Emergence of a New Self-Replicator from a Dynamic Combinatorial Library Requires a Specific Pre-Existing Replicator
- PMID: 28910535
- PMCID: PMC5632813
- DOI: 10.1021/jacs.7b07346
Emergence of a New Self-Replicator from a Dynamic Combinatorial Library Requires a Specific Pre-Existing Replicator
Abstract
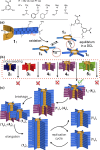
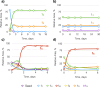
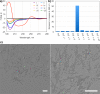
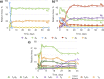
Our knowledge regarding the early steps in the formation of evolvable life and what constitutes the minimal molecular basis of life remains far from complete. The recent emergence of systems chemistry reinvigorated the investigation of systems of self-replicating molecules to address these questions. Most of these studies focus on single replicators and the effects of replicators on the emergence of other replicators remains under-investigated. Here we show the cross-catalyzed emergence of a novel self-replicator from a dynamic combinatorial library made from a threonine containing peptide building block, which, by itself, only forms trimers and tetramers that do not replicate. Upon seeding of this library with different replicators of different macrocycle size (hexamers and octamers), we observed the emergence of hexamer replicator consisting of six units of the threonine peptide only when it is seeded with an octamer replicator containing eight units of a serine building block. These results reveal for the first time how a new replicator can emerge in a process that relies critically on the assistance by another replicator through cross-catalysis and that replicator composition is history dependent.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Cougnon F. B. L.; Sanders J. K. M. Acc. Chem. Res. 2012, 45, 2211–2221. 10.1021/ar200240m. - DOI - PubMed
- Moulin E.; Cormos G.; Giuseppone N. Chem. Soc. Rev. 2012, 41, 1031–1049. 10.1039/C1CS15185A. - DOI - PubMed
- Ladame S. Org. Biomol. Chem. 2008, 6, 219–226. 10.1039/B714599C. - DOI - PubMed
- Corbett P. T.; Leclaire J.; Vial L.; West K. R.; Wietor J.-L.; Sanders J. K. M.; Otto S. Chem. Rev. 2006, 106, 3652–3711. 10.1021/cr020452p. - DOI - PubMed
- Miljanic O. S. Chem. 2017, 2, 502–524. 10.1016/j.chempr.2017.03.002. - DOI
- Jin Y.; Yu C.; Denman R. J.; Zhang W. Chem. Soc. Rev. 2013, 42, 6634–6654. 10.1039/c3cs60044k. - DOI - PubMed
-
- Schrum J. P.; Zhu T. F.; Szostak J. W. Cold Spring Harbor Perspect. Biol. 2010, 2, a002212.10.1101/cshperspect.a002212. - DOI - PMC - PubMed
- Hanczyc M. M. Science 2003, 302, 618–622. 10.1126/science.1089904. - DOI - PMC - PubMed
- Brea R. J.; Hardy M. D.; Devaraj N. K. Chem. - Eur. J. 2015, 21, 12564–12570. 10.1002/chem.201501229. - DOI - PMC - PubMed
- Walde P.; Umakoshi H.; Stano P.; Mavelli F. Chem. Commun. 2014, 50, 10177–10197. 10.1039/C4CC02812K. - DOI - PubMed
-
- Nijemeisland M.; Abdelmohsen L. K. E. A.; Huck W. T. S.; Wilson D. A.; van Hest J. C. M. ACS Cent. Sci. 2016, 2, 843–849. 10.1021/acscentsci.6b00254. - DOI - PMC - PubMed
- Le Saux T.; Plasson R.; Jullien L. Chem. Commun. 2014, 50, 6189–6195. 10.1039/C4CC00392F. - DOI - PubMed
- Hordijk W.; Kauffman S. A.; Steel M. Int. J. Mol. Sci. 2011, 12, 3085–3101. 10.3390/ijms12053085. - DOI - PMC - PubMed
- Keller M.; Kampjut D.; Harrison S.; Ralser M. Nat. Ecol. Evol. 2017, 1, 0083.10.1038/s41559-017-0083. - DOI - PMC - PubMed
-
- Duim H.; Otto S. Beilstein J. Org. Chem. 2017, 13, 1189–1203. 10.3762/bjoc.13.118. - DOI - PMC - PubMed
- Vidonne A.; Philp D. Eur. J. Org. Chem. 2009, 2009, 593–610. 10.1002/ejoc.200800827. - DOI
- Bissette A. J.; Fletcher S. P. Angew. Chem., Int. Ed. 2013, 52, 12800–12826. 10.1002/anie.201303822. - DOI - PubMed
- Clixby G.; Twyman L. Org. Biomol. Chem. 2016, 14, 4170–4184. 10.1039/C6OB00280C. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

