Dopamine Neurons Respond to Errors in the Prediction of Sensory Features of Expected Rewards
- PMID: 28910622
- PMCID: PMC5658021
- DOI: 10.1016/j.neuron.2017.08.025
Dopamine Neurons Respond to Errors in the Prediction of Sensory Features of Expected Rewards
Abstract
Midbrain dopamine neurons have been proposed to signal prediction errors as defined in model-free reinforcement learning algorithms. While these algorithms have been extremely powerful in interpreting dopamine activity, these models do not register any error unless there is a difference between the value of what is predicted and what is received. Yet learning often occurs in response to changes in the unique features that characterize what is received, sometimes with no change in its value at all. Here, we show that classic error-signaling dopamine neurons also respond to changes in value-neutral sensory features of an expected reward. This suggests that dopamine neurons have access to a wider variety of information than contemplated by the models currently used to interpret their activity and that, while their firing may conform to predictions of these models in some cases, they are not restricted to signaling errors in the prediction of value.
Keywords: dopamine; learning; prediction error; rodent; single unit.
Published by Elsevier Inc.
Figures
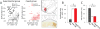

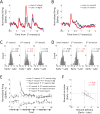
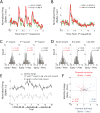

Comment in
-
Error-Driven Learning: Dopamine Signals More Than Value-Based Errors.Curr Biol. 2017 Dec 18;27(24):R1321-R1324. doi: 10.1016/j.cub.2017.10.043. Curr Biol. 2017. PMID: 29257968
References
-
- Brogden WJ. Sensory pre-conditioning. Journal of Experimental Psychology. 1939;25:323–332. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

