Wilted cucumber plants infected by Fusarium oxysporum f. sp. cucumerinum do not suffer from water shortage
- PMID: 28911018
- PMCID: PMC5591412
- DOI: 10.1093/aob/mcx065
Wilted cucumber plants infected by Fusarium oxysporum f. sp. cucumerinum do not suffer from water shortage
Abstract
Background and aims: Fusarium wilt is primarily a soil-borne disease and results in yield loss and quality decline in cucumber (Cucumis sativus). The main symptom of fusarium wilt is the wilting of entire plant, which could be caused by a fungal toxin(s) or blockage of water transport. To investigate whether this wilt arises from water shortage, the physiological responses of hydroponically grown cucumber plants subjected to water stress using polyethylene glycol (PEG, 6000) were compared with those of plants infected with Fusarium oxysporum f. sp. cucumerinum (FOC).
Methods: Parameters reflecting plant water status were measured 8d after the start of treatment. Leaf gas exchange parameters and temperature were measured with a LI-COR portable open photosynthesis system and by thermal imaging. Chlorophyll fluorescence and chloroplast structures were assessed by imaging pulse amplitude-modulated fluorometry and transmission electron microscopy, respectively.
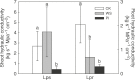
Key results: Cucumber water balance was altered after FOC infection, with decreased water absorption and hydraulic conductivity. However, the responses of cucumber leaves to FOC and PEG differed in leaf regions. Under water stress, measures of lipid peroxidation (malondialdehyde) and chlorophyll fluorescence indicated that the leaf edge was more seriously injured, with a higher leaf temperature and disrupted leaf water status compared with the centre. Here, abscisic acid (ABA) and proline were negatively correlated with water potential. In contrast, under FOC infection, membrane damage and a higher temperature were observed in the leaf centre while ABA and proline did not vary with water potential. Cytologically, FOC-infected cucumber leaves exhibited circular chloroplasts and swelled starch grains in the leaf centre, in which they again differed from PEG-stressed cucumber leaves.
Conclusions: This study illustrates the non-causal relationship between fusarium wilt and water transport blockage. Although leaf wilt occurred in both water stress and FOC infection, the physiological responses were different, especially in leaf spatial distribution.
Keywords: Cucumber (Cucumis sativus); chloroplast; fusarium wilt; injury; leaf regions; temperature; water balance.
© The Author 2017. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For permissions, please email: journals.permissions@oup.com
Figures






References
-
- Aldea M, Hamilton JG, Resti JP. et al. 2006. Comparison of photosynthetic damage from arthropod herbivory and pathogen infection in understory hardwood saplings. Oecologia 149: 221–232. - PubMed
-
- Aldesuquy HS, Abdel-Fattah GM, Baka ZA.. 2000. Changes in chlorophyll, polyamines and chloroplast ultrastructure of Puccinia striiformis induced ‘green islands’ on detached leaves of Triticum aestivum. Plant Physiology and Biochemistry 38: 613–620.
-
- Bates LS, Waldren RP, Teare ID.. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205–207.
-
- Beattie GA. 2011. Water relations in the interaction of foliar bacterial pathogens with plants. Annual Review of Phytopathology 49: 533–555. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

