Homozygous EEF1A2 mutation causes dilated cardiomyopathy, failure to thrive, global developmental delay, epilepsy and early death
- PMID: 28911200
- PMCID: PMC5886049
- DOI: 10.1093/hmg/ddx239
Homozygous EEF1A2 mutation causes dilated cardiomyopathy, failure to thrive, global developmental delay, epilepsy and early death
Abstract
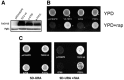
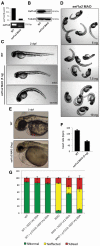
Eukaryotic elongation factor 1A (EEF1A), is encoded by two distinct isoforms, EEF1A1 and EEF1A2; whereas EEF1A1 is expressed almost ubiquitously, EEF1A2 expression is limited such that it is only detectable in skeletal muscle, heart, brain and spinal cord. Currently, the role of EEF1A2 in normal cardiac development and function is unclear. There have been several reports linking de novo dominant EEF1A2 mutations to neurological issues in humans. We report a pair of siblings carrying a homozygous missense mutation p.P333L in EEF1A2 who exhibited global developmental delay, failure to thrive, dilated cardiomyopathy and epilepsy, ultimately leading to death in early childhood. A third sibling also died of a similar presentation, but DNA was unavailable to confirm the mutation. Functional genomic analysis was performed in S. cerevisiae and zebrafish. In S. cerevisiae, there was no evidence for a dominant-negative effect. Previously identified putative de novo mutations failed to complement yeast strains lacking the EEF1A ortholog showing a major growth defect. In contrast, the introduction of the mutation seen in our family led to a milder growth defect. To evaluate its function in zebrafish, we knocked down eef1a2 expression using translation blocking and splice-site interfering morpholinos. EEF1A2-deficient zebrafish had skeletal muscle weakness, cardiac failure and small heads. Human EEF1A2 wild-type mRNA successfully rescued the morphant phenotype, but mutant RNA did not. Overall, EEF1A2 appears to be critical for normal heart function in humans, and its deficiency results in clinical abnormalities in neurologic function as well as in skeletal and cardiac muscle defects.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Lund A., Knudsen S.M., Vissing H., Clark B., Tommerup N. (1996) Assignment of human elongation factor 1alpha genes: EEF1A maps to chromosome 6q14 and EEF1A2 to 20q13.3. Genomics, 36, 359–361. - PubMed
-
- Knudsen S.M., Frydenberg J., Clark B.F., Leffers H. (1993) Tissue-dependent variation in the expression of elongation factor-1 alpha isoforms: isolation and characterisation of a cDNA encoding a novel variant of human elongation-factor 1 alpha. Eur. J. Biochem., 215, 549–554. - PubMed
-
- Shultz L.D., Sweet H.O., Davisson M.T., Coman D.R. (1982) ′Wasted′, a new mutant of the mouse with abnormalities characteristic to ataxia telangiectasia. Nature, 297, 402–404. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

