Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings
- PMID: 28911546
- PMCID: PMC9333423
- DOI: 10.1016/j.jfda.2016.10.017
Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings
Abstract
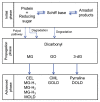
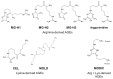
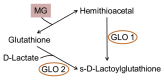
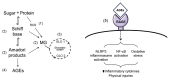
Advanced glycation end products (AGEs) are substances composed of amino groups of proteins and reducing sugars. The initial and propagation phases of the glycation process are accompanied by the production of a large amount of free radicals, carbonyl species, and reactive dicarbonyl species, of which, methylglyoxal (MG) is the most reactive and can cause dicarbonyl stress, influencing normal physiological functions. In the advanced phase, the production of AGEs and the interaction between AGEs and their receptor, RAGE, are also considered to be among the causes of chronic diseases, oxidative stress, and inflammatory reaction. Till date, multiple physiological activities of polyphenols have been confirmed. Recently, there have been many studies discussing the ability of polyphenols to suppress the MG and AGEs formation, which was also confirmed in some in vivo studies. This review article collects recent literatures concerning the effects of polyphenols on the generation of MG and AGEs through different pathways and discusses the feasibility of the inhibition of glycative stress and dicarbonyl stress by polyphenols.
Keywords: advanced glycation end products; antiglycation; flavonoid; phenolic acid; polyphenols.
Copyright © 2016. Published by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Maillard LC. Formation of Melanoidins in a Methodical Way. Compt Rend. 1912;154:66–8.
-
- Peppa M, Vlassara H. Advanced glycation end products and diabetic complications: a general overview. Hormones (Athens) 2005;4:28–37. - PubMed
-
- Yeh WJ, Yang HY, Pai MH, Wu CH, Chen JR. Long-term administration of advanced glycation end product stimulates the activation of NLRP3 inflammasome and sparking the development of renal injury. J Nutr Biochem. 2016;39:68–76. - PubMed
-
- Lin JA, Wu CH, Lu CC, Hsia SM, Yen GC. Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: An emerging biological factor in cancer onset and progression. Mol Nutr Food Res. 2016;60:1850–64. - PubMed
-
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
