Biochemical characterization of a novel antioxidant and angiotensin I-converting enzyme inhibitory peptide from Struthio camelus egg white protein hydrolysis
- PMID: 28911587
- PMCID: PMC9339567
- DOI: 10.1016/j.jfda.2015.11.010
Biochemical characterization of a novel antioxidant and angiotensin I-converting enzyme inhibitory peptide from Struthio camelus egg white protein hydrolysis
Abstract
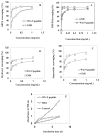
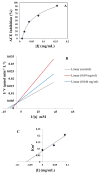
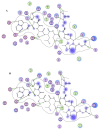
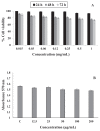
A peptide from ostrich (Struthio camelus) egg white protein hydrolysate (OEWPH) was purified, characterized, and its antioxidant and enzyme inhibitory properties were evaluated. The OEWPH was prepared using pepsin and pancreatin, and then fractionated using reversed-phase high performance liquid chromatography. The antioxidant activity of the WG-9 peptide was investigated based on its scavenging capacity for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, 2,20-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), superoxide (O2•-), hydroxyl (OH•-), and lipid peroxidation inhibition. The angiotensin-converting enzyme (ACE) inhibitory activity and kinetic parameters of the peptide were determined using N-[3-(2-Furyl)acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG) as a substrate. Tandem mass spectrometry analysis of the purified peptide revealed a sequence of WESLSRLLG (MW: 1060 Da; WG-9). This peptide inhibited linoleic acid oxidation and acted as a DPPH (IC50 = 15 ± 0.4 μg/mL), ABTS (IC50 = 130 ± 4.5 μg/mL), superoxide (IC50 = 160 ± 6.4 μg/mL), and hydroxyl (IC50 = 150 ± 6.7 μg/mL) radical scavenger. The ACE-inhibitory activity and kinetic parameters of the WG-9 peptide were determined, showing an ACE inhibitory activity with IC50 of 46.7 ± 1.4 μg/mL. The parameters of peptide/ACE interactions were investigated by molecule docking. Furthermore, viability assays showed that the identified peptide had no cytotoxicity against an HFLF-PI-5 cell line. In conclusion, the WG-9 peptide showed potent antioxidant and ACE-inhibitory activity.
Keywords: angiotensin I-converting enzyme; antioxidant peptide; molecular docking; ostrich egg white proteins.
Copyright © 2016. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Chen HM, Muramoto K, Yamauchi F. Structural analysis of antioxidative peptides from soybean .beta-conglycinin. J Agr Food Chem. 1995;43:574–8.
-
- Hartmann R, Meisel H. Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol. 2007;18:163–9. - PubMed
-
- Herregods G, Van Camp J, Morel N, Ghesquiere B, Gevaert K, Vercruysse L, Vercruysse L, Dierckx S, Quanten E, Smagghe G. Angiotensin I-converting enzyme inhibitory activity of gelatin hydrolysates and identification of bioactive peptides. J Agr Food Chem. 2011;59:552–8. - PubMed
-
- Byun HG, Lee JK, Park HG, Jeon JK, Kim SK. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009;44:842–6.
-
- Miguel M, Manso MA, López-Fandiño R, Ramos M. Comparative study of egg white proteins from different species by chromatographic and electrophoretic methods. Eur Food Res Technol. 2005;221:542–6.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
