Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques
- PMID: 28911634
- PMCID: PMC9328816
- DOI: 10.1016/j.jfda.2016.07.010
Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques
Abstract
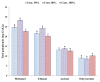
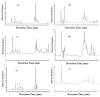
An investigation was carried out to extract polyphenols from the peel of kinnow (Citrus reticulate L.) by maceration and ultrasound-assisted extraction (UAE) techniques. The antioxidant potential of these polyphenols was evaluated using ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and superoxide radical scavenging assays; and their antimicrobial activity was assessed against bacterial strains Staphyloccoccus aureus, Bacillus cereus, and Salmonella typhimurium. The highest extraction yield was obtained through the solvent ethanol at 80% concentration level, whereas UAE was a more efficient technique and yielded comparatively higher polyphenol contents than maceration. Maximum polyphenols were extracted with 80% methanol [32.48 mg gallic acid equivalent (GAE)/g extract] using UAE, whereas minimum phenolics (8.64 mg GAE/g extract) were obtained with 80% ethyl acetate through the maceration technique. Elevated antioxidant activity of kinnow peel extracts was exhibited in three antioxidant assays, where 80% methanolic extracts showed the highest antioxidant activity (27.67±1.11mM/100 g for FRAP) and the highest scavenging activity, 72.83±0.65% and 64.80±0.91% for DPPH and superoxide anion radical assays, respectively. Strong correlations between total polyphenols and antioxidant activity were recorded. Eleven phenolic compounds-including five phenolic acids and six flavonoids-were identified and quantified by high performance liquid chromatography. Ferulic acid and hesperidin were the most abundant compounds whereas caffeic acid was the least abundant phenolic compound in kinnow peel extracts. Maximum inhibition zone was recorded against S. aureus (16.00±0.58 mm) whereas minimum inhibition zone was noted against S. typhimurium (9.00±1.16 mm). It was concluded that kinnow mandarin peels, being a potential source of phenolic compounds with antioxidant and antimicrobial properties, may be used as an ingredient for the preparation of functional foods.
Keywords: antimicrobial activity; antioxidant capacity; kinnow peel; maceration; phenolic compounds; ultrasound.
Copyright © 2016. Published by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



References
-
- Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–9.
-
- Huang CS, Yin MC, Chiu LC. Antihyperglycemic and antioxidative potential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49:2189–95. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
