Macropinocytosis of Bevacizumab by Glioblastoma Cells in the Perivascular Niche Affects their Survival
- PMID: 28912141
- PMCID: PMC5690835
- DOI: 10.1158/1078-0432.CCR-17-0249
Macropinocytosis of Bevacizumab by Glioblastoma Cells in the Perivascular Niche Affects their Survival
Abstract
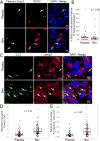
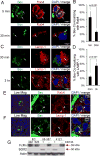
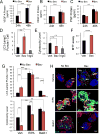
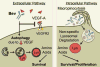
Purpose: Bevacizumab, a humanized monoclonal antibody to VEGF, is used routinely in the treatment of patients with recurrent glioblastoma (GBM). However, very little is known regarding the effects of bevacizumab on the cells in the perivascular space in tumors.Experimental Design: Established orthotopic xenograft and syngeneic models of GBM were used to determine entry of monoclonal anti-VEGF-A into, and uptake by cells in, the perivascular space. Based on the results, we examined CD133+ cells derived from GBM tumors in vitro Bevacizumab internalization, trafficking, and effects on cell survival were analyzed using multilabel confocal microscopy, immunoblotting, and cytotoxicity assays in the presence/absence of inhibitors.Results: In the GBM mouse models, administered anti-mouse-VEGF-A entered the perivascular tumor niche and was internalized by Sox2+/CD44+ tumor cells. In the perivascular tumor cells, bevacizumab was detected in the recycling compartment or the lysosomes, and increased autophagy was found. Bevacizumab was internalized rapidly by CD133+/Sox2+-GBM cells in vitro through macropinocytosis with a fraction being trafficked to a recycling compartment, independent of FcRn, and a fraction to lysosomes. Bevacizumab treatment of CD133+ GBM cells depleted VEGF-A and induced autophagy thereby improving cell survival. An inhibitor of lysosomal acidification decreased bevacizumab-induced autophagy and increased cell death. Inhibition of macropinocytosis increased cell death, suggesting macropinocytosis of bevacizumab promotes CD133+ cell survival.Conclusions: We demonstrate that bevacizumab is internalized by Sox2+/CD44+-GBM tumor cells residing in the perivascular tumor niche. Macropinocytosis of bevacizumab and trafficking to the lysosomes promotes CD133+ cell survival, as does the autophagy induced by bevacizumab depletion of VEGF-A. Clin Cancer Res; 23(22); 7059-71. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures


References
-
- Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7(10):733–6. - PubMed
-
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40. - PubMed
-
- Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9. - PubMed
-
- Nowosielski M, Wiestler B, Goebel G, Hutterer M, Schlemmer HP, Stockhammer G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82(19):1684–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

