Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration
- PMID: 28912156
- PMCID: PMC5656991
- DOI: 10.1523/JNEUROSCI.2085-17.2017
Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration
Abstract
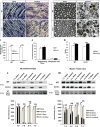
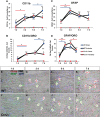
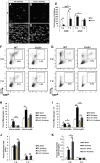
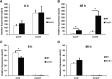
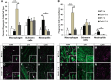
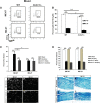
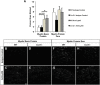
Wallerian degeneration (WD) is considered an essential preparatory stage to the process of axonal regeneration. In the peripheral nervous system, infiltrating monocyte-derived macrophages, which use the chemokine receptor CCR2 to gain entry to injured tissues from the bloodstream, are purportedly necessary for efficient WD. However, our laboratory has previously reported that myelin clearance in the injured sciatic nerve proceeds unhindered in the Ccr2-/- mouse model. Here, we extensively characterize WD in male Ccr2-/- mice and identify a compensatory mechanism of WD that is facilitated primarily by neutrophils. In response to the loss of CCR2, injured Ccr2-/- sciatic nerves demonstrate prolonged expression of neutrophil chemokines, a concomitant extended increase in the accumulation of neutrophils in the nerve, and elevated phagocytosis by neutrophils. Neutrophil depletion substantially inhibits myelin clearance after nerve injury in both male WT and Ccr2-/- mice, highlighting a novel role for these cells in peripheral nerve degeneration that spans genotypes.SIGNIFICANCE STATEMENT The accepted view in the basic and clinical neurosciences is that the clearance of axonal and myelin debris after a nerve injury is directed primarily by inflammatory CCR2+ macrophages. However, we demonstrate that this clearance is nearly identical in WT and Ccr2-/- mice, and that neutrophils replace CCR2+ macrophages as the primary phagocytic cell. We find that neutrophils play a major role in myelin clearance not only in Ccr2-/- mice but also in WT mice, highlighting their necessity during nerve degeneration in the peripheral nervous system. These degeneration studies may propel improvements in nerve regeneration and draw critical parallels to mechanisms of nerve degeneration and regeneration in the CNS and in the context of peripheral neuropathies.
Keywords: Wallerian degeneration; axotomy; macrophages; neutrophils; phagocytosis; sciatic nerve.
Copyright © 2017 the authors 0270-6474/17/3710258-20$15.00/0.
Figures





References
-
- Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, Molina T, Charo I, Hume DA, Cumano A, Lauvau G, Geissmann F (2009) CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med 206:595–606. 10.1084/jem.20081385 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
