Transcription regulation of CDKN1A (p21/CIP1/WAF1) by TRF2 is epigenetically controlled through the REST repressor complex
- PMID: 28912501
- PMCID: PMC5599563
- DOI: 10.1038/s41598-017-11177-1
Transcription regulation of CDKN1A (p21/CIP1/WAF1) by TRF2 is epigenetically controlled through the REST repressor complex
Abstract
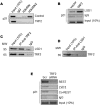
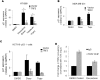
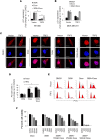
We observed extra-telomeric binding of the telomere repeat binding factor TRF2 within the promoter of the cyclin-dependent kinase CDKNIA (p21/CIP1/WAF1). This result in TRF2 induced transcription repression of p21. Interestingly, p21 repression was through engagement of the REST-coREST-LSD1-repressor complex and altered histone marks at the p21 promoter in a TRF2-dependent fashion. Furthermore, mutational analysis shows p21 repression requires interaction of TRF2 with a p21 promoter G-quadruplex. Physiologically, TRF2-mediated p21 repression attenuated drug-induced activation of cellular DNA damage response by evading G2/M arrest in cancer cells. Together these reveal for the first time role of TRF2 in REST- repressor complex mediated transcription repression.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Telomere length-dependent transcription and epigenetic modifications in promoters remote from telomere ends.PLoS Genet. 2018 Nov 15;14(11):e1007782. doi: 10.1371/journal.pgen.1007782. eCollection 2018 Nov. PLoS Genet. 2018. PMID: 30439955 Free PMC article.
-
Telomere repeat-binding factor 2 binds extensively to extra-telomeric G-quadruplexes and regulates the epigenetic status of several gene promoters.J Biol Chem. 2019 Nov 22;294(47):17709-17722. doi: 10.1074/jbc.RA119.008687. Epub 2019 Oct 1. J Biol Chem. 2019. PMID: 31575660 Free PMC article.
-
Vascular smooth muscle cell-specific regulation of cyclin-dependent kinase inhibitor p21(WAF1/Cip1) transcription by Sp1 is mediated via distinct cis-acting positive and negative regulatory elements in the proximal p21(WAF1/Cip1) promoter.J Cell Biochem. 2004 Nov 15;93(5):904-16. doi: 10.1002/jcb.20238. J Cell Biochem. 2004. PMID: 15389873
-
Decoding transcriptional repressor complexes in the adult central nervous system.Neuropharmacology. 2014 May;80:45-52. doi: 10.1016/j.neuropharm.2013.12.024. Epub 2014 Jan 10. Neuropharmacology. 2014. PMID: 24418103 Free PMC article. Review.
-
Histone deacetylase inhibitors: signalling towards p21cip1/waf1.Int J Biochem Cell Biol. 2007;39(7-8):1367-74. doi: 10.1016/j.biocel.2007.03.001. Epub 2007 Mar 7. Int J Biochem Cell Biol. 2007. PMID: 17412634 Review.
Cited by
-
The Intra- and Extra-Telomeric Role of TRF2 in the DNA Damage Response.Int J Mol Sci. 2021 Sep 14;22(18):9900. doi: 10.3390/ijms22189900. Int J Mol Sci. 2021. PMID: 34576063 Free PMC article. Review.
-
The regulation and functions of DNA and RNA G-quadruplexes.Nat Rev Mol Cell Biol. 2020 Aug;21(8):459-474. doi: 10.1038/s41580-020-0236-x. Epub 2020 Apr 20. Nat Rev Mol Cell Biol. 2020. PMID: 32313204 Free PMC article. Review.
-
Telomere length-dependent transcription and epigenetic modifications in promoters remote from telomere ends.PLoS Genet. 2018 Nov 15;14(11):e1007782. doi: 10.1371/journal.pgen.1007782. eCollection 2018 Nov. PLoS Genet. 2018. PMID: 30439955 Free PMC article.
-
Non-duplex G-Quadruplex DNA Structure: A Developing Story from Predicted Sequences to DNA Structure-Dependent Epigenetics and Beyond.Acc Chem Res. 2021 Jan 5;54(1):46-56. doi: 10.1021/acs.accounts.0c00431. Epub 2020 Dec 21. Acc Chem Res. 2021. PMID: 33347280 Free PMC article.
-
The role of p21/CIP1/WAF1 (p21) in the negative regulation of the growth hormone/growth hormone receptor and epidermal growth factor/epidermal growth factor receptor pathways, in growth hormone transduction defect.Ann Pediatr Endocrinol Metab. 2018 Dec;23(4):204-209. doi: 10.6065/apem.2018.23.4.204. Epub 2018 Dec 31. Ann Pediatr Endocrinol Metab. 2018. PMID: 30599481 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

