Oral fibroblasts modulate the macrophage response to bacterial challenge
- PMID: 28912533
- PMCID: PMC5599598
- DOI: 10.1038/s41598-017-11771-3
Oral fibroblasts modulate the macrophage response to bacterial challenge
Abstract
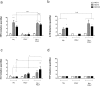
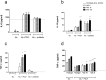
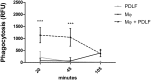
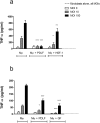
Tissue damage in chronic periodontal disease is driven by the host response to a dysbiotic microbiota, and not by bacteria directly. Among chronic inflammatory diseases of the oral cavity, inflammation and tissue damage around dental implants (peri-implantitis) is emerging as a major clinical challenge, since it is more severe and less responsive to treatment compared to inflammation around natural teeth. We tested whether oral fibroblasts from the periodontal ligament (PDLF), which are present around natural teeth but not around dental implants, actively regulate inflammatory responses to bacterial stimulation. We show that human PDLF down-regulate TNF-α post-transcriptionally in macrophages stimulated with the oral pathogen Porphyromonas gingivalis. Cell contact and secretion of IL-6 and IL-10 contribute to the modulation of inflammatory cytokine production. Although fibroblasts decreased TNF-α secretion, they enhanced the ability of macrophages to phagocytose bacteria. Surprisingly, donor matched oral fibroblasts from gingival tissues, or fibroblasts from peri-implant inflamed tissues were at least as active as PDLF in regulating macrophage responses to bacteria. In addition, priming fibroblasts with inflammatory mediators enhanced PDLF regulatory activity. A further understanding of the spectrum of fibroblast activities in inflammatory lesions is important in order to design ways to control inflammatory tissue damage.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Gingival and periodontal ligament fibroblasts differ in their inflammatory response to viable Porphyromonas gingivalis.J Periodontal Res. 2010 Apr;45(2):262-70. doi: 10.1111/j.1600-0765.2009.01229.x. Epub 2009 Sep 23. J Periodontal Res. 2010. PMID: 19778323
-
Unprimed, M1 and M2 Macrophages Differentially Interact with Porphyromonas gingivalis.PLoS One. 2016 Jul 6;11(7):e0158629. doi: 10.1371/journal.pone.0158629. eCollection 2016. PLoS One. 2016. PMID: 27383471 Free PMC article.
-
Influence of titanium on in vitro fibroblast-Porphyromonas gingivalis interaction in peri-implantitis.J Clin Periodontol. 2013 Sep;40(9):841-9. doi: 10.1111/jcpe.12136. Epub 2013 Jul 22. J Clin Periodontol. 2013. PMID: 23875835
-
Inhibition of NF-κB by Pyrrolidine Dithiocarbamate Prevents the Inflammatory Response in a Ligature-Induced Peri-Implantitis Model: A Canine Study.Cell Physiol Biochem. 2018;49(2):610-625. doi: 10.1159/000492997. Epub 2018 Aug 30. Cell Physiol Biochem. 2018. PMID: 30165363
-
Fibroblasts and macrophages: Collaborators in tissue homeostasis.Immunol Rev. 2021 Jul;302(1):86-103. doi: 10.1111/imr.12989. Epub 2021 Jun 8. Immunol Rev. 2021. PMID: 34101202 Review.
Cited by
-
Fibroblasts at the curtain call: from ensemble to principal dancers in immunometabolism and inflammaging.J Appl Oral Sci. 2023 Jun 23;31:e20230050. doi: 10.1590/1678-7757-2023-0050. eCollection 2023. J Appl Oral Sci. 2023. PMID: 37377310 Free PMC article. Review.
-
Viability and collagen secretion by fibroblasts on titanium surfaces with different acid-etching protocols.Int J Implant Dent. 2019 Nov 21;5(1):41. doi: 10.1186/s40729-019-0192-4. Int J Implant Dent. 2019. PMID: 31749041 Free PMC article.
-
A Review of Antimicrobial Activity of Dental Mesenchymal Stromal Cells: Is There Any Potential?Front Oral Health. 2022 Jan 14;2:832976. doi: 10.3389/froh.2021.832976. eCollection 2021. Front Oral Health. 2022. PMID: 35098213 Free PMC article. Review.
-
The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis.J Dent Res. 2023 May;102(5):489-496. doi: 10.1177/00220345231151921. Epub 2023 Mar 8. J Dent Res. 2023. PMID: 36883660 Free PMC article. Review.
-
ROS-Induced Gingival Fibroblast Senescence: Implications in Exacerbating Inflammatory Responses in Periodontal Disease.Inflammation. 2024 Dec;47(6):1918-1935. doi: 10.1007/s10753-024-02014-5. Epub 2024 Apr 17. Inflammation. 2024. PMID: 38630168
References
-
- Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. Journal of periodontology. 2003;74(6):6–35. - PubMed
-
- Normand S, et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9601–9606. doi: 10.1073/pnas.1100981108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

