Matrisome Profiling During Intervertebral Disc Development And Ageing
- PMID: 28912585
- PMCID: PMC5599645
- DOI: 10.1038/s41598-017-11960-0
Matrisome Profiling During Intervertebral Disc Development And Ageing
Abstract
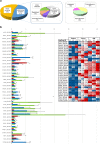
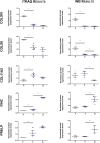
Intervertebral disc (IVD) degeneration is often the cause of low back pain. Degeneration occurs with age and is accompanied by extracellular matrix (ECM) depletion, culminating in nucleus pulpous (NP) extrusion and IVD destruction. The changes that occur in the disc with age have been under investigation. However, a thorough study of ECM profiling is needed, to better understand IVD development and age-associated degeneration. As so, iTRAQ LC-MS/MS analysis of foetus, young and old bovine NPs, was performed to define the NP matrisome. The enrichment of Collagen XII and XIV in foetus, Fibronectin and Prolargin in elder NPs and Collagen XI in young ones was independently validated. This study provides the first matrisome database of healthy discs during development and ageing, which is key to determine the pathways and processes that maintain disc homeostasis. The factors identified may help to explain age-associated IVD degeneration or constitute putative effectors for disc regeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

