Perspectives for biocatalytic lignin utilization: cleaving 4- O-5 and Cα-Cβ bonds in dimeric lignin model compounds catalyzed by a promiscuous activity of tyrosinase
- PMID: 28912833
- PMCID: PMC5594458
- DOI: 10.1186/s13068-017-0900-3
Perspectives for biocatalytic lignin utilization: cleaving 4- O-5 and Cα-Cβ bonds in dimeric lignin model compounds catalyzed by a promiscuous activity of tyrosinase
Abstract
Background: In the biorefinery utilizing lignocellulosic biomasses, lignin decomposition to value-added phenolic derivatives is a key issue, and recently biocatalytic delignification is emerging owing to its superior selectivity, low energy consumption, and unparalleled sustainability. However, besides heme-containing peroxidases and laccases, information about lignolytic biocatalysts is still limited till date.
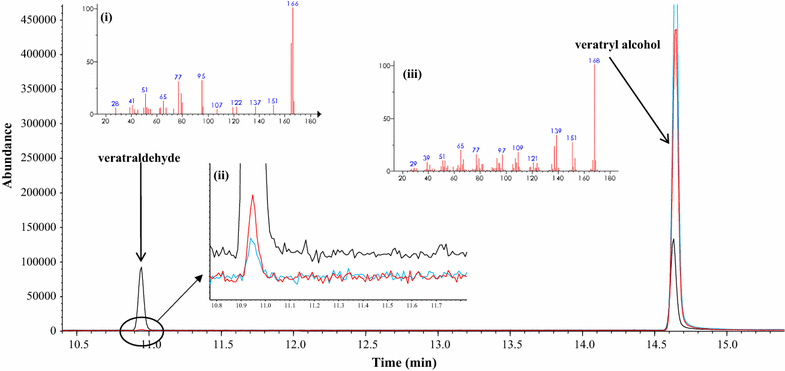
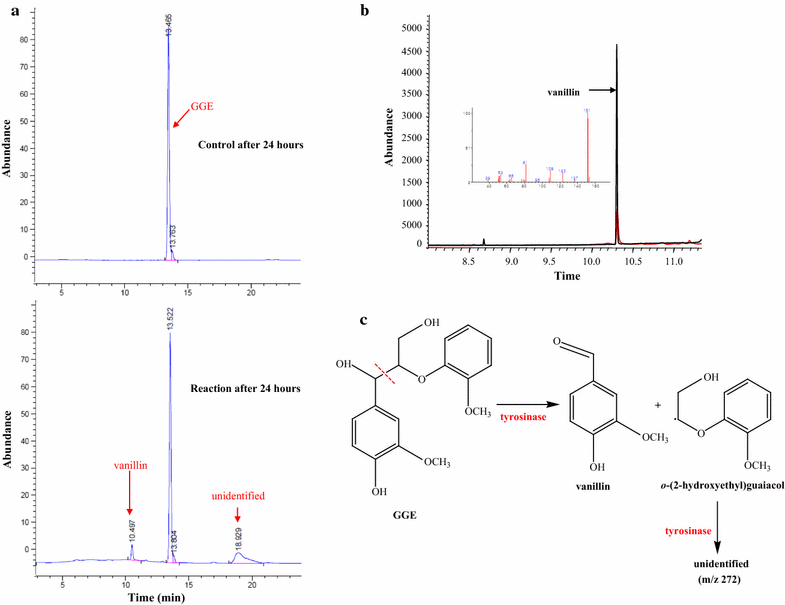
Results: Herein, we report a promiscuous activity of tyrosinase which is closely associated with delignification requiring high redox potentials (>1.4 V vs. normal hydrogen electrode [NHE]). The promiscuous activity of tyrosinase not only oxidizes veratryl alcohol, a commonly used nonphenolic substrate for assaying ligninolytic activity, to veratraldehyde but also cleaves the 4-O-5 and Cα-Cβ bonds in 4-phenoxyphenol and guaiacyl glycerol-β-guaiacyl ether (GGE) that are dimeric lignin model compounds. Cyclic voltammograms additionally verified that the promiscuous activity oxidizes lignin-related high redox potential substrates.
Conclusion: These results might be applicable for extending the versatility of tyrosinase toward biocatalytic delignification as well as suggesting a new perspective for sustainable lignin utilization. Furthermore, the results provide insight for exploring the previously unknown promiscuous activities of biocatalysts much more diverse than ever thought before, thereby innovatively expanding the applicable area of biocatalysis.
Keywords: 4-Phenoxyphenol; Guaiacyl glycerol-β-guaiacyl ether (GGE); Promiscuous activity; Sustainable lignin utilization; Tyrosinase.
Figures


Similar articles
-
A dye-decolorizing peroxidase from Bacillus subtilis exhibiting substrate-dependent optimum temperature for dyes and β-ether lignin dimer.Sci Rep. 2015 Feb 4;5:8245. doi: 10.1038/srep08245. Sci Rep. 2015. PMID: 25650125 Free PMC article.
-
Phenolic mediators enhance the manganese peroxidase catalyzed oxidation of recalcitrant lignin model compounds and synthetic lignin.Fungal Genet Biol. 2014 Nov;72:137-149. doi: 10.1016/j.fgb.2014.07.008. Epub 2014 Aug 7. Fungal Genet Biol. 2014. PMID: 25108071
-
Characterization and use of a bacterial lignin peroxidase with an improved manganese-oxidative activity.Appl Microbiol Biotechnol. 2018 Dec;102(24):10579-10588. doi: 10.1007/s00253-018-9409-3. Epub 2018 Oct 9. Appl Microbiol Biotechnol. 2018. PMID: 30302519
-
Structure and action mechanism of ligninolytic enzymes.Appl Biochem Biotechnol. 2009 May;157(2):174-209. doi: 10.1007/s12010-008-8279-z. Epub 2008 Jun 26. Appl Biochem Biotechnol. 2009. PMID: 18581264 Review.
-
From gene to biorefinery: microbial β-etherases as promising biocatalysts for lignin valorization.Front Microbiol. 2015 Sep 4;6:916. doi: 10.3389/fmicb.2015.00916. eCollection 2015. Front Microbiol. 2015. PMID: 26388858 Free PMC article. Review.
Cited by
-
Hemocyanin facilitates lignocellulose digestion by wood-boring marine crustaceans.Nat Commun. 2018 Dec 3;9(1):5125. doi: 10.1038/s41467-018-07575-2. Nat Commun. 2018. PMID: 30510200 Free PMC article.
-
Effects of Lignin-Diverted Reductant with Polyphenol Oxidases on Cellulose Degradation by Wild and Mutant Types of Lytic Polysaccharide Monooxygenase.Curr Issues Mol Biol. 2024 Apr 21;46(4):3694-3712. doi: 10.3390/cimb46040230. Curr Issues Mol Biol. 2024. PMID: 38666960 Free PMC article.
-
A multi-omics approach to lignocellulolytic enzyme discovery reveals a new ligninase activity from Parascedosporium putredinis NO1.Proc Natl Acad Sci U S A. 2021 May 4;118(18):e2008888118. doi: 10.1073/pnas.2008888118. Proc Natl Acad Sci U S A. 2021. PMID: 33903229 Free PMC article.
-
In silico exploration of lignin peroxidase for unraveling the degradation mechanism employing lignin model compounds.RSC Adv. 2021 Apr 20;11(24):14632-14653. doi: 10.1039/d0ra10840e. eCollection 2021 Apr 15. RSC Adv. 2021. PMID: 35423962 Free PMC article.
-
Secretory Production of the Hericium erinaceus Laccase from Saccharomyces cerevisiae.J Microbiol Biotechnol. 2024 Apr 28;34(4):930-939. doi: 10.4014/jmb.2312.12043. Epub 2024 Jan 22. J Microbiol Biotechnol. 2024. PMID: 38314447 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

