A crossover study of the combination therapy of metformin and exenatide or biphasic insulin aspart 30 in overweight or obese patients newly diagnosed with type 2 diabetes mellitus
- PMID: 28912879
- PMCID: PMC5585756
- DOI: 10.3892/etm.2017.4863
A crossover study of the combination therapy of metformin and exenatide or biphasic insulin aspart 30 in overweight or obese patients newly diagnosed with type 2 diabetes mellitus
Abstract
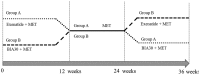
The aim of the present study was to explore the effects of various combinations of exenatide, metformin (MET) and biphasic insulin aspart 30 (BIA30) on type 2 diabetes mellitus (T2DM). Two hundred overweight or obese patients newly diagnosed with T2DM were evenly randomized into two groups: A (twice daily for all: Phase I, 5 µg exenatide + 0.5 g MET for 4 weeks, then 10 µg exenatide + 0.5 g MET for 8 weeks; Phase II, 0.5 g MET for 12 weeks; Phase III, 0.3-0.4 U/kg/day BIA30 + 0.5 g MET for 12 weeks) and B (Phases I, II, III matched the phases III, II and I in group A). In groups A and B a significant decrease and increase, respectively, in glycated hemoglobin (HbAlc) and body mass index (BMI) was noted during Phase I. A 3.2±0.4-kg decrease in body weight in group A and a 2.6±0.3-kg increase in group B was observed. In Phase II, HbAlc was significantly increased in both groups (P<0.05). In Phase III, the BMI was increased in group A and reduced in group B (P<0.05). There was a 3.8±0.4-kg weight decrease in group B and 4.2±0.5-kg increase in group A (P<0.05). The combination of exenatide and MET promoted weight loss, glycemic control, β-cell function index, C peptide and adiponectin levels. These results suggested that the combination of exenatide and MET is better than the combination of BIA and MET for the therapy of overweight or obese patients newly diagnosed with T2DM.
Keywords: biphasic insulin aspart; combination therapy; exenatide; metformin; obesity; overweight; type 2 diabetes mellitus.
Figures
Similar articles
-
Phase III Study on Efficacy and Safety of Triple Combination (Exenatide/Metformin/Biphasic Insulin Aspart) Therapy for Type 2 Diabetes Mellitus.Am J Ther. 2018 Nov/Dec;25(6):e609-e616. doi: 10.1097/MJT.0000000000000431. Am J Ther. 2018. PMID: 26844723 Clinical Trial.
-
Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea.Curr Med Res Opin. 2009 Jan;25(1):65-75. doi: 10.1185/03007990802597951. Curr Med Res Opin. 2009. PMID: 19210140 Clinical Trial.
-
Comparison of Blood Glucose Variability Between Exenatide and Biphasic Insulin Aspart 30 in Chinese Participants with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy: A Multicenter, Open-Label, Randomized Trial.Diabetes Ther. 2020 Oct;11(10):2313-2328. doi: 10.1007/s13300-020-00904-z. Epub 2020 Aug 27. Diabetes Ther. 2020. PMID: 32856226 Free PMC article.
-
Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea).Drugs. 2007;67(6):935-54. doi: 10.2165/00003495-200767060-00008. Drugs. 2007. PMID: 17428109 Review.
-
Targeting the pathophysiology of type 2 diabetes: rationale for combination therapy with pioglitazone and exenatide.Curr Med Res Opin. 2008 Nov;24(11):3009-22. doi: 10.1185/03007990802390795. Epub 2008 Oct 2. Curr Med Res Opin. 2008. PMID: 18828960 Review.
Cited by
-
Comparison of changes in adipokine and inflammatory cytokine levels in patients with newly diagnosed type 2 diabetes treated with exenatide, insulin, or pioglitazone: A post-hoc study of the CONFIDENCE trial.Heliyon. 2023 Dec 5;10(1):e23309. doi: 10.1016/j.heliyon.2023.e23309. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38169889 Free PMC article.
-
Impact of Incretin-Based Therapies on Adipokines and Adiponectin.J Diabetes Res. 2021 Oct 7;2021:3331865. doi: 10.1155/2021/3331865. eCollection 2021. J Diabetes Res. 2021. PMID: 34660808 Free PMC article. Review.
-
Efficacy of Exenatide Administered Twice Daily in Body Mass Index Reduction in Patients with Type 2 Diabetes Mellitus.Int J Clin Pract. 2022 Oct 10;2022:7128859. doi: 10.1155/2022/7128859. eCollection 2022. Int J Clin Pract. 2022. PMID: 37214201 Free PMC article. Clinical Trial.
References
-
- Knudsen Bjerre L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
