Haemoprotozoa: Making biological sense of molecular phylogenies
- PMID: 28913164
- PMCID: PMC5582378
- DOI: 10.1016/j.ijppaw.2017.08.007
Haemoprotozoa: Making biological sense of molecular phylogenies
Abstract
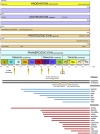
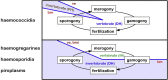
A range of protistan parasites occur in the blood of vertebrates and are transmitted by haematophagous invertebrate vectors. Some 48 genera are recognized in bood primarily on the basis of parasite morphology and host specificity; including extracellular kinetoplastids (trypanosomatids) and intracellular apicomplexa (haemogregarines, haemococcidia, haemosporidia and piroplasms). Gene sequences are available for a growing number of species and molecular phylogenies often link parasite and host or vector evolution. This review endeavours to reconcile molecular clades with biological characters. Four major trypanosomatid clades have been associated with site of development in the vector: salivarian or stercorarian for Trypanosoma, and supra- or peri-pylorian for Leishmania. Four haemogregarine clades have been associated with acarine vectors (Hepatozoon A and B, Karyolysus, Hemolivia) and another two with leeches (Dactylosoma, Haemogregarina sensu stricto). Two haemococcidian clades (Lankesterella, Schellackia) using leeches and mosquitoes (as paratenic hosts!) were paraphyletic with monoxenous enteric coccidia. Two major haemosporidian clades have been associated with mosquito vectors (Plasmodium from mammals, Plasmodium from birds and lizards), two with midges (Hepatocystis from bats, Parahaemoproteus from birds) and two with louse-flies and black-flies (Haemoproteus and Leucocytozoon from birds). Three major piroplasm clades were recognized: one associated with transovarian transmission in ticks (Babesia sensu stricto); one with pre-erythrocytic schizogony in vertebrates (Theileria/Cytauxzoon); and one with neither (Babesia sensu lato). Broad comparative studies with allied groups suggest that trypanosomatids and haemogregarines evolved first in aquatic and then terrestrial environments, as evidenced by extant lineages in invertebrates and their radiation in vertebrates. In contrast, haemosporidia and haemococcidia are thought to have evolved first in vertebrates from proto-coccidia and then incorporated invertebrate vectors. Piroplasms are thought to have evolved in ticks and diversified into mammals. More molecular studies are required on more parasite taxa to refine current thought, but ultimately transmission studies are mandated to determine the vectors for many haemoprotozoa.
Keywords: Biology; Haemoprotozoa; Invertebrate vectors; Phylogeny; Vertebrate hosts.
Figures









References
-
- Adl S.M., Simpson A.G.B., Lane C.E., Lukes J., Bass D., Bowser S.S., Brown M.W., Burki F., Dunthorn M., Hampl V., Heiss A., Hoppenrath M., Lara E., Le Gall L., Lynn D.H., McManus H., Mitchell E.A.D., Mozley-Stanridge S.E., Parfrey L.W., Pawlowski J., Rueckert S., Shadwick L., Schoch C.L., Smirnov A., Spiegel F.W. The revised classification of eukaryotes. J. Euk. Microbiol. 2012;59:429–493. - PMC - PubMed
-
- Arisue N., Hashimoto T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol. Int. 2015;64:254–259. - PubMed
-
- Asato Y., Oshiro M., Myint C.K., Yamamoto Y., Kato H., Marco J.D., Mimori T., Gomez E.A.L., Hashiguchi Y., Uezato H. Phylogenetic analysis of the genus Leishmania by cytochrome b gene sequencing. Expt. Parasitol. 2009;121:352–361. - PubMed
-
- Balasubramaniam S., Mulder R.A., Sunnucks P., Pavlova A., Amos J.N., Melville J. Prevalence and diversity of avian haematozoa in three species of Australian passerine. Emu. 2013;113:353–358.
-
- Barta J.R., Ogedengbe J.D., Martin D.S., Smith T.G. Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18S rDNA sequences. J. Euk. Microbiol. 2012;59:171–180. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

