Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma
- PMID: 28913336
- PMCID: PMC5583162
- DOI: 10.3389/fmed.2017.00135
Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma
Abstract
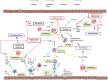
The definition of asthma has changed considerably in recent years, to the extent that asthma is no longer considered a single disease but a heterogeneous disorder that includes several phenotypes and, possibly, endotypes. A more detailed analysis of the immunological mechanisms underlying the pathogenesis of asthma shows interleukin 5 (IL-5) to be a crucial cytokine in several asthma phenotypes. In fact, IL-5 exerts selective action on eosinophils, which, in turn, sustain airway inflammation and worsen asthma symptoms and control. Clinical trials have shown drugs targeting IL-5 or its receptor alpha subunit (IL-5Ra) to be a promising therapeutic approach to severe asthma, whose characteristics render standard therapy of little use: systemic corticosteroids only partially control the disease and have well-known adverse effects, and omalizumab is used for allergic subtypes. Analysis of the design process of clinical trials reveals the importance of patient selection, taking into account both clinical data (e.g., exacerbations, lung function, and quality of life) and biomarkers (e.g., eosinophils, which are predictive of therapeutic response).
Keywords: biomarkers; eosinophils; interleukin 5; monoclonal antibodies; personalized medicine; precision medicine; safety; severe asthma.
Figures
Similar articles
-
[Clinical and economic analysis of Reslizumab use in the treatment of patients with severe allergic eosinophilic asthma].Ter Arkh. 2019 Dec 15;91(12):47-56. doi: 10.26442/00403660.2019.12.000452. Ter Arkh. 2019. PMID: 32598589 Russian.
-
Targeting the interleukin-4 and interleukin-13 pathways in severe asthma: current knowledge and future needs.Curr Opin Pulm Med. 2018 Jan;24(1):50-55. doi: 10.1097/MCP.0000000000000436. Curr Opin Pulm Med. 2018. PMID: 29036019 Review.
-
A recombinant humanized anti-IgE monoclonal antibody (omalizumab) in the therapy of moderate-to-severe allergic asthma.Recent Pat Inflamm Allergy Drug Discov. 2007 Nov;1(3):225-31. doi: 10.2174/187221307782418900. Recent Pat Inflamm Allergy Drug Discov. 2007. PMID: 19075985 Review.
-
Omalizumab as alternative to chronic use of oral corticosteroids in severe asthma.Respir Med. 2019 Apr;150:51-62. doi: 10.1016/j.rmed.2019.02.003. Epub 2019 Feb 7. Respir Med. 2019. PMID: 30961951 Review.
-
Antibody therapy for the management of severe asthma with eosinophilic inflammation.Int Immunol. 2017 Jul 1;29(7):337-343. doi: 10.1093/intimm/dxx045. Int Immunol. 2017. PMID: 28910970 Review.
Cited by
-
The use of biologics for immune modulation in allergic disease.J Clin Invest. 2019 Mar 18;129(4):1452-1462. doi: 10.1172/JCI124607. eCollection 2019 Mar 18. J Clin Invest. 2019. PMID: 30882368 Free PMC article. Review.
-
Treatable Mechanisms in Asthma.Mol Diagn Ther. 2021 Mar;25(2):111-121. doi: 10.1007/s40291-021-00514-w. Epub 2021 Feb 11. Mol Diagn Ther. 2021. PMID: 33570719 Free PMC article.
-
Gender dimorphism in IgA subclasses in T2-high asthma.Clin Exp Med. 2023 Jul;23(3):929-941. doi: 10.1007/s10238-022-00828-x. Epub 2022 Apr 25. Clin Exp Med. 2023. PMID: 35467314 Free PMC article.
-
Eosinophilic Granulomatosis With Polyangiitis: Newer Therapies.Curr Rheumatol Rep. 2018 Apr 2;20(5):23. doi: 10.1007/s11926-018-0736-2. Curr Rheumatol Rep. 2018. PMID: 29611001 Review.
-
Tartaric Acid Exacerbates DSS-Induced Colitis by Promoting Eosinophilic Inflammation via IL-13 and IL-5Rα Upregulation.Pathogens. 2025 Apr 8;14(4):366. doi: 10.3390/pathogens14040366. Pathogens. 2025. PMID: 40333150 Free PMC article.
References
-
- Global Initiative for the Management of Asthma 2015. (2016). Available from: www.ginasthma.org
-
- Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol (2011) 127:382–9.10.1016/j.jaci.2010.11.015 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources