New Insights into the Pathogenesis of Celiac Disease
- PMID: 28913337
- PMCID: PMC5583152
- DOI: 10.3389/fmed.2017.00137
New Insights into the Pathogenesis of Celiac Disease
Abstract
Celiac disease (CD) is an autoimmune and multisystem gluten-related disorder that causes symptoms involving the gastrointestinal tract and other organs. Pathogenesis of CD is only partially known. It had been established that ingestion of gluten proteins present in wheat and other cereals are necessary for the disease and develops in individuals genetically predisposed carrying the DQ2 or DQ8 human leukocyte antigen haplotypes. In this review, we had pay specific attention on the last discoveries regarding the three cellular components mainly involved in the development and maintenance of CD: T-cells, B-cells, and microbioma. All of them had been showed critical for the interaction between inflammatory immune response and gluten peptides. Although the mechanisms of interaction among overall these components are not yet fully understood, recent proteomics and molecular studies had shed some lights in the pathogenic role of tissue transglutaminase 2 in CD and in the alteration of the intestinal barrier function induced by host microbiota.
Keywords: B-cell; celiac disease; immunoglobulin; microbioma; transglutaminase.
Figures

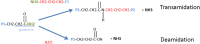



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

