Periostin in vitreoretinal diseases
- PMID: 28913545
- PMCID: PMC11107734
- DOI: 10.1007/s00018-017-2651-5
Periostin in vitreoretinal diseases
Abstract
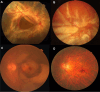
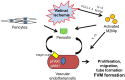
Proliferative vitreoretinal diseases such as diabetic retinopathy, proliferative vitreoretinopathy (PVR), and age-related macular degeneration are a leading cause of decreased vision and blindness in developed countries. In these diseases, retinal fibro(vascular) membrane (FVM) formation above and beneath the retina plays an important role. Gene expression profiling of human FVMs revealed significant upregulation of periostin. Subsequent analyses demonstrated increased periostin expression in the vitreous of patients with both proliferative diabetic retinopathy and PVR. Immunohistochemical analysis showed co-localization of periostin with α-SMA and M2 macrophage markers in FVMs. In vitro, periostin blockade inhibited migration and adhesion induced by PVR vitreous and transforming growth factor-β2 (TGF-β2). In vivo, a novel single-stranded RNAi agent targeting periostin showed the inhibitory effect on experimental retinal and choroidal FVM formation without affecting the viability of retinal cells. These results indicated that periostin is a pivotal molecule for FVM formation and a promising therapeutic target for these proliferative vitreoretinal diseases.
Keywords: Age-related macular degeneration; Choroid; Epiretinal membranes; Fibrosis; Fibrovascular membranes; Genome-wide gene expression profiling; Mouse model of laser-induced choroidal neovascuarization; Mouse model of oxygen-induced retinal neovascularization; Neovascularization; Proliferative diabetic retinopathy; Proliferative vitreoretinopathy; Retina; Single-stranded RNA interference; Vitreoretinal disease.
Figures


References
-
- Yoshida S. Identification of molecular targets for intraocular proliferative diseases using genomicapproaches. J Jpn Ophthalmol Soc. 2014;118:241–282.
-
- Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Zhou Y, Nakao S, Sassa Y, Oshima Y, Takao K, Shimahara A, Yoshikawa K, Hamasaki T, Ohgi T, Hayashi H, Matsuda A, Kudo A, Nozaki M, Ogura Y, Kuroda M, Ishibashi T. Inhibition of choroidal fibrovascular membrane formation by new class of rna interference therapeutic agent targeting periostin. Gene Ther. 2015;22:127–137. doi: 10.1038/gt.2014.112. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

