Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury
- PMID: 28913553
- PMCID: PMC6287604
- DOI: 10.1007/s00395-017-0652-z
Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury
Abstract
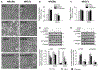
Early reperfusion of ischemic cardiac tissue increases inflammatory cell infiltration which contributes to cardiomyocyte death and loss of cardiac function, referred to as ischemia/reperfusion (IR) injury. Neutrophil- and mast cell-derived proteases, cathepsin G (Cat.G) and chymase, are released early after IR, but their function is complicated by potentially redundant actions and targets. This study investigated whether a dual inhibition of Cat.G and chymase influences cardiomyocyte injury and wound healing after experimental IR in mice. Treatment with a dual Cat.G and chymase inhibitor (DCCI) immediately after reperfusion blocked cardiac Cat.G and chymase activity induced after IR, which resulted in decreased immune response in the infarcted heart. Mice treated with DCCI had less myocardial collagen deposition and showed preserved ventricular function at 1 and 7 days post-IR compared with vehicle-treated mice. DCCI treatment also significantly attenuated focal adhesion (FA) complex disruption and myocyte degeneration after IR. Treatment of isolated cardiomyocytes with Cat.G or chymase significantly promoted FA signaling downregulation, myofibril degeneration and myocyte apoptosis. Conversely, treatment of cardiac fibroblasts with Cat.G or chymase induced FA signaling activation and increased their migration and differentiation to myofibroblasts. These opposite responses in cardiomyocytes and fibroblasts were blocked by treatment with DCCI. These findings show that Cat.G and chymase are key mediators of myocyte apoptosis and fibroblast migration and differentiation that play a role in adverse cardiac remodeling and function post-IR. Thus, dual targeting of neutrophil- and mast cell-derived proteases could be used as a novel therapeutic strategy to reduce post-IR inflammation and improve cardiac remodeling.
Keywords: Cardioprotection; Inflammation; Inflammatory serine proteases; Myocardial ischemia–reperfusion.
Conflict of interest statement
Figures


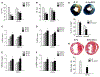



References
-
- Beghdadi W, Madjene LC, Benhamou M, Charles N, Gautier G, Launay P, Blank U (2011) Mast cells as cellular sensors in inflammation and immunity. Front Immunol 2:37. doi:10.3389/fimmu.2011.00037 - DOI - PMC - PubMed
-
- Caughey GH (2016) Mast cell proteases as pharmacological targets. Eur J Pharmacol 778:44–55. doi:10.1016/j.ejphar.2015.04.045 - DOI - PMC - PubMed
-
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT (2000) Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Investig 106:55–62. doi:10.1172/JCI8768 - DOI - PMC - PubMed
-
- Frangogiannis NG (2012) Regulation of the inflammatory response in cardiac repair. Circ Res 110:159–173. doi:10.1161/CIRCRESAHA.111.243162 - DOI - PMC - PubMed
-
- Fu L, Wei C-C, Powell PC, Bradley WE, Ahmad S, Ferrario CM, Collawn JF, Dell’Italia LJ (2016) Increased fibroblast chymase production mediates procollagen autophagic digestion in volume overload. J Mol Cell Cardiol 92:1–9. doi:10.1016/j.yjmcc.2016.01.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

