Liver-specific reconstitution of CEACAM1 reverses the metabolic abnormalities caused by its global deletion in male mice
- PMID: 28913658
- PMCID: PMC5788286
- DOI: 10.1007/s00125-017-4432-y
Liver-specific reconstitution of CEACAM1 reverses the metabolic abnormalities caused by its global deletion in male mice
Abstract
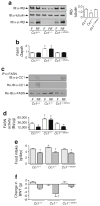
Aims/hypothesis: The carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) promotes insulin clearance. Mice with global null mutation (Cc1 -/-) or with liver-specific inactivation (L-SACC1) of Cc1 (also known as Ceacam1) gene display hyperinsulinaemia resulting from impaired insulin clearance, insulin resistance, steatohepatitis and obesity. Because increased lipolysis contributes to the metabolic phenotype caused by transgenic inactivation of CEACAM1 in the liver, we aimed to further investigate the primary role of hepatic CEACAM1-dependent insulin clearance in insulin and lipid homeostasis. To this end, we examined whether transgenic reconstitution of CEACAM1 in the liver of global Cc1 -/- mutant mice reverses their abnormal metabolic phenotype.
Methods: Insulin response was assessed by hyperinsulinaemic-euglycaemic clamp analysis and energy balance was analysed by indirect calorimetry. Mice were overnight-fasted and refed for 7 h to assess fatty acid synthase activity in the liver and the hypothalamus in response to insulin release during refeeding.
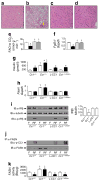
Results: Liver-based rescuing of CEACAM1 restored insulin clearance, plasma insulin level, insulin sensitivity and steatohepatitis caused by global deletion of Cc1. It also reversed the gain in body weight and total fat mass observed with Cc1 deletion, in parallel to normalising energy balance. Mechanistically, reversal of hyperphagia appeared to result from reducing fatty acid synthase activity and restoring insulin signalling in the hypothalamus.
Conclusions/interpretation: Despite the potential confounding effects of deleting Cc1 from extrahepatic tissues, liver-based rescuing of CEACAM1 resulted in full normalisation of the metabolic phenotype, underscoring the key role that CEACAM1-dependent hepatic insulin clearance pathways play in regulating systemic insulin sensitivity, lipid homeostasis and energy balance.
Keywords: CEACAM1; Energy balance; Fatty acid synthase; Hyperinsulinaemia; Insulin clearance; Insulin resistance; Lipolysis; Normoinsulinaemia; Steatohepatitis.
Conflict of interest statement
Figures


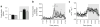

Similar articles
-
Hyperinsulinemia drives hepatic insulin resistance in male mice with liver-specific Ceacam1 deletion independently of lipolysis.Metabolism. 2019 Apr;93:33-43. doi: 10.1016/j.metabol.2019.01.008. Epub 2019 Jan 19. Metabolism. 2019. PMID: 30664851 Free PMC article.
-
Liver-specific rescuing of CEACAM1 reverses endothelial and cardiovascular abnormalities in male mice with null deletion of Ceacam1 gene.Mol Metab. 2018 Mar;9:98-113. doi: 10.1016/j.molmet.2018.01.009. Epub 2018 Jan 31. Mol Metab. 2018. PMID: 29396368 Free PMC article.
-
Carcinoembryonic antigen-related cell adhesion molecule 1: a link between insulin and lipid metabolism.Diabetes. 2008 Sep;57(9):2296-303. doi: 10.2337/db08-0379. Epub 2008 Jun 10. Diabetes. 2008. PMID: 18544705 Free PMC article.
-
Loss of Hepatic CEACAM1: A Unifying Mechanism Linking Insulin Resistance to Obesity and Non-Alcoholic Fatty Liver Disease.Front Endocrinol (Lausanne). 2017 Jan 26;8:8. doi: 10.3389/fendo.2017.00008. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28184213 Free PMC article. Review.
-
CEACAM1 in Liver Injury, Metabolic and Immune Regulation.Int J Mol Sci. 2018 Oct 11;19(10):3110. doi: 10.3390/ijms19103110. Int J Mol Sci. 2018. PMID: 30314283 Free PMC article. Review.
Cited by
-
Regulation of hepatic fibrosis by carcinoembryonic antigen-related cell adhesion molecule 1.Metabolism. 2021 Aug;121:154801. doi: 10.1016/j.metabol.2021.154801. Epub 2021 May 28. Metabolism. 2021. PMID: 34058224 Free PMC article.
-
Hyperinsulinemia drives hepatic insulin resistance in male mice with liver-specific Ceacam1 deletion independently of lipolysis.Metabolism. 2019 Apr;93:33-43. doi: 10.1016/j.metabol.2019.01.008. Epub 2019 Jan 19. Metabolism. 2019. PMID: 30664851 Free PMC article.
-
Role of lncRNA LIPE-AS1 in adipogenesis.Adipocyte. 2022 Dec;11(1):11-27. doi: 10.1080/21623945.2021.2013415. Adipocyte. 2022. PMID: 34957921 Free PMC article.
-
Alternative splicing of CEACAM1 by hypoxia-inducible factor-1α enhances tolerance to hepatic ischemia in mice and humans.Sci Transl Med. 2023 Aug 2;15(707):eadf2059. doi: 10.1126/scitranslmed.adf2059. Epub 2023 Aug 2. Sci Transl Med. 2023. PMID: 37531413 Free PMC article.
-
Heart Uptake of [18F]Fluoro-4-Thia-Oleate in a Non-Alcoholic Fatty Liver Disease Mouse Model.Pharmaceuticals (Basel). 2022 Dec 17;15(12):1577. doi: 10.3390/ph15121577. Pharmaceuticals (Basel). 2022. PMID: 36559027 Free PMC article.
References
-
- Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–S268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

