Reporting to Improve Reproducibility and Facilitate Validity Assessment for Healthcare Database Studies V1.0
- PMID: 28913963
- PMCID: PMC5639362
- DOI: 10.1002/pds.4295
Reporting to Improve Reproducibility and Facilitate Validity Assessment for Healthcare Database Studies V1.0
Erratum in
-
Erratum.Pharmacoepidemiol Drug Saf. 2017 Dec;26(12):1570. doi: 10.1002/pds.4353. Pharmacoepidemiol Drug Saf. 2017. PMID: 29205684 Free PMC article. No abstract available.
Abstract
Purpose: Defining a study population and creating an analytic dataset from longitudinal healthcare databases involves many decisions. Our objective was to catalogue scientific decisions underpinning study execution that should be reported to facilitate replication and enable assessment of validity of studies conducted in large healthcare databases.
Methods: We reviewed key investigator decisions required to operate a sample of macros and software tools designed to create and analyze analytic cohorts from longitudinal streams of healthcare data. A panel of academic, regulatory, and industry experts in healthcare database analytics discussed and added to this list.
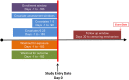
Conclusion: Evidence generated from large healthcare encounter and reimbursement databases is increasingly being sought by decision-makers. Varied terminology is used around the world for the same concepts. Agreeing on terminology and which parameters from a large catalogue are the most essential to report for replicable research would improve transparency and facilitate assessment of validity. At a minimum, reporting for a database study should provide clarity regarding operational definitions for key temporal anchors and their relation to each other when creating the analytic dataset, accompanied by an attrition table and a design diagram. A substantial improvement in reproducibility, rigor and confidence in real world evidence generated from healthcare databases could be achieved with greater transparency about operational study parameters used to create analytic datasets from longitudinal healthcare databases.
Keywords: Transparency; healthcare databases; longitudinal data; methods; pharmacoepidemiology; replication; reproducibility.
© 2017 The Authors. Pharmacoepidemiology & Drug Safety Published by John Wiley & Sons Ltd.
Figures
Comment in
-
Guidance to reinforce the credibility of health care database studies and ensure their appropriate impact.Pharmacoepidemiol Drug Saf. 2017 Sep;26(9):1013-1017. doi: 10.1002/pds.4305. Pharmacoepidemiol Drug Saf. 2017. PMID: 28913965 No abstract available.
References
-
- Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. Apr 2005;58(4):323‐337. - PubMed
-
- Califf RM, Robb MA, Bindman AB, et al. Transforming Evidence Generation to Support Health and Health Care Decisions. N Engl J Med. 2016;375(24):2395‐2400. - PubMed
-
- Psaty BM, Breckenridge AM. Mini‐Sentinel and regulatory science‐‐big data rendered fit and functional. N Engl J Med. Jun 5. 2014;370(23):2165‐2167. - PubMed
-
- Oliveira JL, Lopes P, Nunes T, et al. The EU‐ADR Web Platform: delivering advanced pharmacovigilance tools. Pharmacoepidemiol Drug Saf. 2013 May. 2013;22(5):459‐467. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical