Diffuse Staining for Activated NOTCH1 Correlates With NOTCH1 Mutation Status and Is Associated With Worse Outcome in Adenoid Cystic Carcinoma
- PMID: 28914715
- PMCID: PMC5657508
- DOI: 10.1097/PAS.0000000000000945
Diffuse Staining for Activated NOTCH1 Correlates With NOTCH1 Mutation Status and Is Associated With Worse Outcome in Adenoid Cystic Carcinoma
Abstract
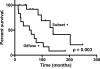
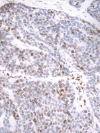
NOTCH1 is frequently mutated in adenoid cystic carcinoma (ACC). To test the idea that immunohistochemical (IHC) staining can identify ACCs with NOTCH1 mutations, we performed IHC for activated NOTCH1 (NICD1) in 197 cases diagnosed as ACC from 173 patients. NICD1 staining was positive in 194 cases (98%) in 2 major patterns: subset positivity, which correlated with tubular/cribriform histology; and diffuse positivity, which correlated with a solid histology. To determine the relationship between NICD1 staining and NOTCH1 mutational status, targeted exome sequencing data were obtained on 14 diffusely NICD1-positive ACC specimens from 11 patients and 15 subset NICD1-positive ACC specimens from 15 patients. This revealed NOTCH1 gain-of-function mutations in 11 of 14 diffusely NICD1-positive ACC specimens, whereas all subset-positive tumors had wild-type NOTCH1 alleles. Notably, tumors with diffuse NICD1 positivity were associated with significantly worse outcomes (P=0.003). To determine whether NOTCH1 activation is unique among tumors included in the differential diagnosis with ACC, we performed NICD1 IHC on a cohort of diverse salivary gland and head and neck tumors. High fractions of each of these tumor types were positive for NICD1 in a subset of cells, particularly in basaloid squamous cell carcinomas; however, sequencing of basaloid squamous cell carcinomas failed to identify NOTCH1 mutations. These findings indicate that diffuse NICD1 positivity in ACC correlates with solid growth pattern, the presence of NOTCH1 gain-of-function mutations, and unfavorable outcome, and suggest that staining for NICD1 can be helpful in distinguishing ACC with solid growth patterns from other salivary gland and head and neck tumors.
Conflict of interest statement
The authors have nothing to disclose and declare no conflicts of interest.
Figures


Similar articles
-
Deciphering NOTCH1 as a Biomarker in Adenoid Cystic Carcinoma: Insights From a Systematic Review With Meta-Analysis.J Oral Pathol Med. 2025 Sep;54(8):647-657. doi: 10.1111/jop.70028. Epub 2025 Aug 11. J Oral Pathol Med. 2025. PMID: 40789681 Free PMC article. Review.
-
Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors.J Clin Oncol. 2017 Jan 20;35(3):352-360. doi: 10.1200/JCO.2016.67.5264. Epub 2016 Nov 21. J Clin Oncol. 2017. PMID: 27870570 Free PMC article.
-
Morphological heterogeneity of oral salivary gland carcinomas: a clinicopathologic study of 41 cases with long term follow-up emphasizing the overlapping spectrum of adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma.Int J Clin Exp Pathol. 2011 Apr;4(4):336-48. Epub 2011 Apr 18. Int J Clin Exp Pathol. 2011. PMID: 21577319 Free PMC article.
-
Detection of MYB Alterations and Other Immunohistochemical Markers in Primary Cutaneous Adenoid Cystic Carcinoma.Am J Surg Pathol. 2015 Oct;39(10):1347-56. doi: 10.1097/PAS.0000000000000463. Am J Surg Pathol. 2015. PMID: 26076064
-
The value of MYB as a prognostic marker for adenoid cystic carcinoma: Meta-analysis.Head Neck. 2019 May;41(5):1517-1524. doi: 10.1002/hed.25597. Epub 2019 Feb 13. Head Neck. 2019. PMID: 30759319 Review.
Cited by
-
MicroRNA dysregulation in adenoid cystic carcinoma of the salivary gland in relation to prognosis and gene fusion status: a cohort study.Virchows Arch. 2018 Sep;473(3):329-340. doi: 10.1007/s00428-018-2423-0. Epub 2018 Aug 1. Virchows Arch. 2018. PMID: 30069755
-
Deciphering NOTCH1 as a Biomarker in Adenoid Cystic Carcinoma: Insights From a Systematic Review With Meta-Analysis.J Oral Pathol Med. 2025 Sep;54(8):647-657. doi: 10.1111/jop.70028. Epub 2025 Aug 11. J Oral Pathol Med. 2025. PMID: 40789681 Free PMC article. Review.
-
Prognostic significance of 1p36 locus deletion in adenoid cystic carcinoma of the salivary glands.Virchows Arch. 2018 Oct;473(4):471-480. doi: 10.1007/s00428-018-2349-6. Epub 2018 Apr 4. Virchows Arch. 2018. PMID: 29619555
-
Prognostic Impact of Notch1 Intracellular Domain, P63, and c-MYC in Lacrimal Gland Adenoid Cystic Carcinoma.Invest Ophthalmol Vis Sci. 2024 Sep 3;65(11):4. doi: 10.1167/iovs.65.11.4. Invest Ophthalmol Vis Sci. 2024. PMID: 39230995 Free PMC article.
-
The Therapeutic Landscape of Salivary Gland Malignancies-Where Are We Now?Int J Mol Sci. 2022 Nov 28;23(23):14891. doi: 10.3390/ijms232314891. Int J Mol Sci. 2022. PMID: 36499216 Free PMC article. Review.
References
-
- Nascimento AG, Amaral AL, Prado LA, Kligerman J, Silveira TR. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer. 1986;57(2):312–9. - PubMed
-
- da Cruz Perez DE, de Abreu Alves F, Nobuko Nishimoto I, de Almeida OP, Kowalski LP. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol. 2006;42(2):139–46. - PubMed
-
- Bjorndal K, Krogdahl A, Therkildsen MH, Charabi B, Kristensen CA, Andersen E, et al. Salivary adenoid cystic carcinoma in Denmark 1990–2005: Outcome and independent prognostic factors including the benefit of radiotherapy. Results of the Danish Head and Neck Cancer Group (DAHANCA) Oral Oncol. 2015;51(12):1138–42. - PubMed
-
- van Weert S, van der Waal I, Witte BI, Leemans CR, Bloemena E. Histopathological grading of adenoid cystic carcinoma of the head and neck: analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 2015;51(1):71–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

