MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and α1,3-fucosylation
- PMID: 28914881
- PMCID: PMC5686352
- DOI: 10.1038/cdd.2017.136
MicroRNA-200c impairs uterine receptivity formation by targeting FUT4 and α1,3-fucosylation
Abstract
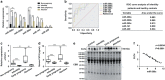
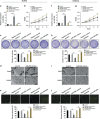
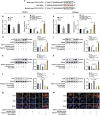
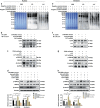
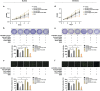
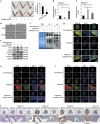
Successful embryo implantation requires the establishment of a receptive endometrium. Poor endometrial receptivity has generally been considered as a major cause of infertility. Protein glycosylation is associated with many physiological and pathological processes. The fucosylation is catalyzed by the specific fucosyltransferases. Fucosyltransferase IV (FUT4) is the key enzyme for the biosynthesis of α1,3-fucosylated glycans carried by glycoproteins, and the previous studies showed FUT4 expression changed dynamically during perimplantation. MicroRNAs (miRNAs) are known to regulate specific gene expression. However, the relationship between specific miRNA and FUT4, as well as the role of miRNA/FUT4 in the establishment of uterine receptivity remains elusive. In the current study, we reported that the levels of miR-200 family members were significantly increased in serum from infertility and abortion patients relative to healthy non-pregnancy and early-pregnancy women. Among these, miR-200c was the most sensitive diagnostic criterion for infertility by receiver operating characteristic curve analysis. FUT4 was lower in the serum from infertility and abortion patients compared with the healthy non-pregnancy and early-pregnancy women. Using endometrial cell lines and a mouse model, we demonstrated that miR-200c targeted and inhibited FUT4 expression, leading to the dysfunction of uterine receptivity. Our results also revealed that miR-200c decreased α1.3-fucosylation on glycoprotein CD44, which further inactivated Wnt/β-catenin signaling pathway. Taken together, miR-200c hampers uterine receptivity formation by targeting FUT4 and α1.3-fucosylation on CD44. miR-200c and FUT4 may be applied together as the potential markers for endometrial receptivity, and useful diagnostic and therapeutic targets for infertility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bartosch C, Lopes JM, Beires J, Sousa M. Human endometrium ultrastructure during the implantation window: a new perspective of the epithelium cell types. Reprod Sci 2011; 18: 525–539. - PubMed
-
- Salamonsen LA, Evans J, Nguyen HP, Edgell TA. The microenvironment of human Implantation: determinant of reproductive success. Am J Reprod Immunol 2016; 75: 218–225. - PubMed
-
- Riad ON, Hak AA. Assessment of endometrial receptivity using Doppler ultrasonography in infertile women undergoing intrauterine insemination. Gynecol Endocrinol 2014; 30: 70–73. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

