SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress
- PMID: 28914882
- PMCID: PMC5762839
- DOI: 10.1038/cdd.2017.144
SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress
Abstract
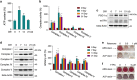
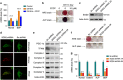
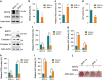
Recent studies have revealed robust metabolic changes during cell differentiation. Mitochondria, the organelles where many vital metabolic reactions occur, may play an important role. Here, we report the involvement of SIRT3-regulated mitochondrial stress in osteoblast differentiation and bone formation. In both the osteoblast cell line MC3T3-E1 and primary calvarial osteoblasts, robust mitochondrial biogenesis and supercomplex formation were observed during differentiation, accompanied by increased ATP production and decreased mitochondrial stress. Inhibition of mitochondrial activity or an increase in mitochondrial superoxide production significantly suppressed osteoblast differentiation. During differentiation, SOD2 was specifically induced to eliminate excess mitochondrial superoxide and protein oxidation, whereas SIRT3 expression was increased to enhance SOD2 activity through deacetylation of K68. Both SOD2 and SIRT3 knockdown resulted in suppression of differentiation. Meanwhile, mice deficient in SIRT3 exhibited obvious osteopenia accompanied by osteoblast dysfunction, whereas overexpression of SOD2 or SIRT3 improved the differentiation capability of primary osteoblasts derived from SIRT3-deficient mice. These results suggest that SIRT3/SOD2 is required for regulating mitochondrial stress and plays a vital role in osteoblast differentiation and bone formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 2011; 13: 27–38. - PubMed
-
- Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer 2016; 16: 373–386. - PubMed
-
- Kassem M, Bianco P. Skeletal stem cells in space and time. Cell 2015; 160: 17–19. - PubMed
-
- Kain KH, Popov VL, Herzog NK. Alterations in mitochondria and mtTFA in response to LPS-induced differentiation of B-cells. Bioch Biophys Acta 2000; 1494: 91–103. - PubMed
-
- San Martin N, Cervera AM, Cordova C, Covarello D, McCreath KJ, Galvez BG. Mitochondria determine the differentiation potential of cardiac mesoangioblasts. Stem Cells 2011; 29: 1064–1074. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

