Targeting Hypoxia-Inducible Factor-1α/Pyruvate Dehydrogenase Kinase 1 Axis by Dichloroacetate Suppresses Bleomycin-induced Pulmonary Fibrosis
- PMID: 28915065
- PMCID: PMC5805994
- DOI: 10.1165/rcmb.2016-0186OC
Targeting Hypoxia-Inducible Factor-1α/Pyruvate Dehydrogenase Kinase 1 Axis by Dichloroacetate Suppresses Bleomycin-induced Pulmonary Fibrosis
Abstract
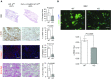
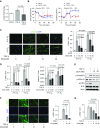
Hypoxia has long been implicated in the pathogenesis of fibrotic diseases. Aberrantly activated myofibroblasts are the primary pathological driver of fibrotic progression, yet how various microenvironmental influences, such as hypoxia, contribute to their sustained activation and differentiation is poorly understood. As a defining feature of hypoxia is its impact on cellular metabolism, we sought to investigate how hypoxia-induced metabolic reprogramming affects myofibroblast differentiation and fibrotic progression, and to test the preclinical efficacy of targeting glycolytic metabolism for the treatment of pulmonary fibrosis. Bleomycin-induced pulmonary fibrotic progression was evaluated in two independent, fibroblast-specific, promoter-driven, hypoxia-inducible factor (Hif) 1A knockout mouse models and in glycolytic inhibitor, dichloroacetate-treated mice. Genetic and pharmacological approaches were used to explicate the role of metabolic reprogramming in myofibroblast differentiation. Hypoxia significantly enhanced transforming growth factor-β-induced myofibroblast differentiation through HIF-1α, whereas overexpression of the critical HIF-1α-mediated glycolytic switch, pyruvate dehydrogenase kinase 1 (PDK1) was sufficient to activate glycolysis and potentiate myofibroblast differentiation, even in the absence of HIF-1α. Inhibition of the HIF-1α/PDK1 axis by genomic deletion of Hif1A or pharmacological inhibition of PDK1 significantly attenuated bleomycin-induced pulmonary fibrosis. Our findings suggest that HIF-1α/PDK1-mediated glycolytic reprogramming is a critical metabolic alteration that acts to promote myofibroblast differentiation and fibrotic progression, and demonstrate that targeting glycolytic metabolism may prove to be a potential therapeutic strategy for the treatment of pulmonary fibrosis.
Keywords: dichloroacetate; fibroblasts; hypoxia inducible factor-1α; pulmonary fibrosis; pyruvate dehydrogenase kinase1.
Figures





References
-
- American Thoracic Society: idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

