Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure
- PMID: 28915070
- PMCID: PMC6047068
- DOI: 10.1080/01913123.2017.1362088
Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure
Abstract
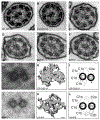
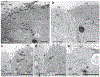
Primary ciliary dyskinesia (PCD) is a genetic disorder causing chronic oto-sino-pulmonary disease. No single diagnostic test will detect all PCD cases. Transmission electron microscopy (TEM) of respiratory cilia was previously considered the gold standard diagnostic test for PCD, but 30% of all PCD cases have either normal ciliary ultrastructure or subtle changes which are non-diagnostic. These cases are identified through alternate diagnostic tests, including nasal nitric oxide measurement, high-speed videomicroscopy analysis, immunofluorescent staining of axonemal proteins, and/or mutation analysis of various PCD causing genes. Autosomal recessive mutations in DNAH11 and HYDIN produce normal TEM ciliary ultrastructure, while mutations in genes encoding for radial spoke head proteins result in some cross-sections with non-diagnostic alterations in the central apparatus interspersed with normal ciliary cross-sections. Mutations in nexin link and dynein regulatory complex genes lead to a collection of different ciliary ultrastructures; mutations in CCDC65, CCDC164, and GAS8 produce normal ciliary ultrastructure, while mutations in CCDC39 and CCDC40 cause absent inner dynein arms and microtubule disorganization in some ciliary cross-sections. Mutations in CCNO and MCIDAS cause near complete absence of respiratory cilia due to defects in generation of multiple cellular basal bodies; however, the scant cilia generated may have normal ultrastructure. Lastly, a syndromic form of PCD with retinal degeneration results in normal ciliary ultrastructure through mutations in the RPGR gene. Clinicians must be aware of these genetic causes of PCD resulting in non-diagnostic TEM ciliary ultrastructure and refrain from using TEM of respiratory cilia as a test to rule out PCD.
Keywords: Electron microscopy; PCD; genetic testing; primary ciliary dyskinesia.
Conflict of interest statement
Declaration: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Figures
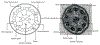
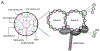




Similar articles
-
[Cilia ultrastructural and gene variation of primary ciliary dyskinesia: report of three cases and literatures review].Zhonghua Er Ke Za Zhi. 2018 Feb 2;56(2):134-137. doi: 10.3760/cma.j.issn.0578-1310.2018.02.012. Zhonghua Er Ke Za Zhi. 2018. PMID: 29429202 Review. Chinese.
-
CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia.PLoS One. 2013 Aug 26;8(8):e72299. doi: 10.1371/journal.pone.0072299. eCollection 2013. PLoS One. 2013. PMID: 23991085 Free PMC article.
-
Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure.Thorax. 2012 May;67(5):433-41. doi: 10.1136/thoraxjnl-2011-200301. Epub 2011 Dec 18. Thorax. 2012. PMID: 22184204 Free PMC article.
-
Mutations in GAS8, a Gene Encoding a Nexin-Dynein Regulatory Complex Subunit, Cause Primary Ciliary Dyskinesia with Axonemal Disorganization.Hum Mutat. 2016 Aug;37(8):776-85. doi: 10.1002/humu.23005. Epub 2016 May 12. Hum Mutat. 2016. PMID: 27120127
-
Transmission electron microscopy in the diagnosis of primary ciliary dyskinesia.Ups J Med Sci. 2006;111(1):155-68. doi: 10.3109/2000-1967-010. Ups J Med Sci. 2006. PMID: 16553254 Review.
Cited by
-
The RSPH4A Gene in Primary Ciliary Dyskinesia.Int J Mol Sci. 2023 Jan 18;24(3):1936. doi: 10.3390/ijms24031936. Int J Mol Sci. 2023. PMID: 36768259 Free PMC article. Review.
-
Thalamic Neuron Resilience during Osmotic Demyelination Syndrome (ODS) Is Revealed by Primary Cilium Outgrowth and ADP-ribosylation factor-like protein 13B Labeling in Axon Initial Segment.Int J Mol Sci. 2023 Nov 17;24(22):16448. doi: 10.3390/ijms242216448. Int J Mol Sci. 2023. PMID: 38003639 Free PMC article.
-
HY-DIN' in the Cilia: Discovery of Central Pair-related Mutations in Primary Ciliary Dyskinesia.Am J Respir Cell Mol Biol. 2020 Mar;62(3):281-282. doi: 10.1165/rcmb.2019-0316ED. Am J Respir Cell Mol Biol. 2020. PMID: 31604022 Free PMC article. No abstract available.
-
A protein complex in the extreme distal tip of vertebrate motile cilia controls their organization, length, and function.bioRxiv [Preprint]. 2025 Jun 25:2025.02.19.639145. doi: 10.1101/2025.02.19.639145. bioRxiv. 2025. PMID: 40027778 Free PMC article. Preprint.
-
Rare Human Diseases: Model Organisms in Deciphering the Molecular Basis of Primary Ciliary Dyskinesia.Cells. 2019 Dec 11;8(12):1614. doi: 10.3390/cells8121614. Cells. 2019. PMID: 31835861 Free PMC article. Review.
References
-
- Kouis P, Yiallouros PK, Middleton N, Evans JS, Kyriacou K, Papatheodorou SI. Prevalence of primary ciliary dyskinesia in consecutive referrals of suspect cases and the transmission electron microscopy detection rate: a systematic review and meta-analysis. Pediatr Res. 2017;81(3):398–405. - PubMed
-
- Papon JF, Coste A, Roudot-Thoraval F, et al. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2010;35(5):1057–1063. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
