Inflammatory cytokine production in tumor cells upon chemotherapy drug exposure or upon selection for drug resistance
- PMID: 28915246
- PMCID: PMC5600395
- DOI: 10.1371/journal.pone.0183662
Inflammatory cytokine production in tumor cells upon chemotherapy drug exposure or upon selection for drug resistance
Abstract
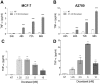
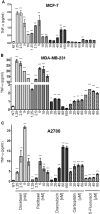
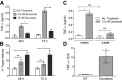
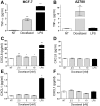
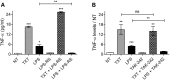
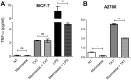
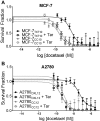
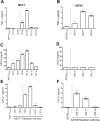
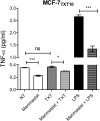
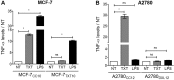
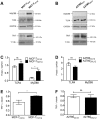
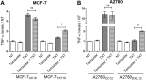
Tumor Necrosis Factor alpha (TNF-α) has been shown to be released by tumor cells in response to docetaxel, and lipopolysaccharides (LPS), the latter through activation of toll-like receptor 4 (TLR4). However, it is unclear whether the former involves TLR4 receptor activation through direct binding of the drug to TLR4 at the cell surface. The current study was intended to better understand drug-induced TNF-α production in tumor cells, whether from short-term drug exposure or in cells selected for drug resistance. ELISAs were employed to measure cytokine release from breast and ovarian tumor cells in response to several structurally distinct chemotherapy agents and/or TLR4 agonists or antagonists. Drug uptake and drug sensitivity studies were also performed. We observed that several drugs induced TNF-αrelease from multiple tumor cell lines. Docetaxel-induced cytokine production was distinct from that of LPS in both MyD88-positive (MCF-7) and MyD88-deficient (A2780) cells. The acquisition of docetaxel resistance was accompanied by increased constitutive production of TNF-αand CXCL1, which waned at higher levels of resistance. In docetaxel-resistant MCF-7 and A2780 cell lines, the production of TNF-α could not be significantly augmented by docetaxel without the inhibition of P-gp, a transporter protein that promotes drug efflux from tumor cells. Pretreatment of tumor cells with LPS sensitized MyD88-positive cells (but not MyD88-deficient) to docetaxel cytotoxicity in both drug-naive and drug-resistant cells. Our findings suggest that taxane-induced inflammatory cytokine production from tumor cells depends on the duration of exposure, requires cellular drug-accumulation, and is distinct from the LPS response seen in breast tumor cells. Also, stimulation of the LPS-induced pathway may be an attractive target for treatment of drug-resistant disease.
Conflict of interest statement
Figures


Similar articles
-
Alterations in tumor necrosis factor signaling pathways are associated with cytotoxicity and resistance to taxanes: a study in isogenic resistant tumor cells.Breast Cancer Res. 2012 Jan 6;14(1):R2. doi: 10.1186/bcr3083. Breast Cancer Res. 2012. PMID: 22225778 Free PMC article.
-
Toll-like receptor 4 ligation confers chemoresistance to docetaxel on PC-3 human prostate cancer cells.Cell Biol Toxicol. 2012 Aug;28(4):269-77. doi: 10.1007/s10565-012-9221-2. Epub 2012 Jun 1. Cell Biol Toxicol. 2012. PMID: 22648782
-
Endotoxin tolerance attenuates LPS-induced TLR4 mobilization to lipid rafts: a condition reversed by PKC activation.J Leukoc Biol. 2006 Dec;80(6):1289-97. doi: 10.1189/jlb.0106053. Epub 2006 Sep 7. J Leukoc Biol. 2006. PMID: 16959900
-
Sensitivity of docetaxel-resistant MCF-7 breast cancer cells to microtubule-destabilizing agents including vinca alkaloids and colchicine-site binding agents.PLoS One. 2017 Aug 7;12(8):e0182400. doi: 10.1371/journal.pone.0182400. eCollection 2017. PLoS One. 2017. PMID: 28787019 Free PMC article.
-
[Biological characteristics of drug induced tumor cells and its medicine prevention and treatment].Yao Xue Xue Bao. 2016 Feb;51(2):182-8. Yao Xue Xue Bao. 2016. PMID: 29856197 Review. Chinese.
Cited by
-
Age-related divergence of circulating immune responses in patients with solid tumors treated with immune checkpoint inhibitors.Nat Commun. 2025 Apr 21;16(1):3531. doi: 10.1038/s41467-025-58512-z. Nat Commun. 2025. PMID: 40258833 Free PMC article.
-
Docetaxel Administration via Novel Hierarchical Nanoparticle Reduces Proinflammatory Cytokine Levels in Prostate Cancer Cells.Cancers (Basel). 2025 May 23;17(11):1758. doi: 10.3390/cancers17111758. Cancers (Basel). 2025. PMID: 40507238 Free PMC article.
-
Polarization of Macrophages in Insects: Opening Gates for Immuno-Metabolic Research.Front Cell Dev Biol. 2021 Feb 15;9:629238. doi: 10.3389/fcell.2021.629238. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33659253 Free PMC article. Review.
-
Interruption of the long non-coding RNA HOTAIR signaling axis ameliorates chemotherapy-induced cachexia in bladder cancer.J Biomed Sci. 2022 Dec 6;29(1):104. doi: 10.1186/s12929-022-00887-y. J Biomed Sci. 2022. PMID: 36471329 Free PMC article.
-
Tumor-produced immune regulatory factors as a therapeutic target in cancer treatment.Front Immunol. 2024 Aug 14;15:1416458. doi: 10.3389/fimmu.2024.1416458. eCollection 2024. Front Immunol. 2024. PMID: 39206193 Free PMC article. Review. No abstract available.
References
-
- Centers for Disease Control and Prevention n.d. http://www.cdc.gov/cancer/international/statistics.htm (accessed January 7, 2016).
-
- GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012 n.d. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed January 7, 2016).
-
- Smith I, Chua S. Medical treatment of early breast cancer. I: adjuvant treatment. BMJ 2006;332:34–7. doi: 10.1136/bmj.332.7532.34 - DOI - PMC - PubMed
-
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown a, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672–85. doi: 10.1200/JCO.1998.16.8.2672 - DOI - PubMed
-
- Eltahir A, Heys SD, Hutcheon AW, Sarkar TK, Smith I, Walker LG, et al. Treatment of large and locally advanced breast cancers using neoadjuvant chemotherapy. Am J Surg 1998;175:127–32. S0002961097002791 [pii]. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

