Long-term efficacy and safety of rituximab in IgG4-related disease: Data from a French nationwide study of thirty-three patients
- PMID: 28915275
- PMCID: PMC5600376
- DOI: 10.1371/journal.pone.0183844
Long-term efficacy and safety of rituximab in IgG4-related disease: Data from a French nationwide study of thirty-three patients
Abstract
Objectives: To assess efficacy and safety of rituximab (RTX) as induction therapy, maintenance of remission and treatment of relapses in a cohort of IgG4-related disease (IgG4-RD) patients.
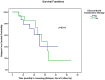
Methods: Nationwide retrospective multicenter study of IgG4-RD patients treated with at least one course of RTX. Clinical, biological and radiological response, relapse rate and drug tolerance were analyzed. Kaplan-Meier curves were plotted and risk factors for relapse studied with a Cox regression model.
Results: Among 156 IgG4-RD patients included in the French database, 33 received rituximab. Clinical response was noted in 29/31 (93.5%) symptomatic patients. Glucocorticoids withdrawal was achieved in 17 (51.5%) patients. During a mean follow-up of 24.8 ±21 months, 13/31 (41.9%) responder patients relapsed after a mean delay of 19 ±11 months after RTX. Active disease, as defined by an IgG4-RD Responder Index >9 before RTX, was significantly associated with relapse (HR = 3.68, 95% CI: 1.1, 12.6) (P = 0.04), whereas maintenance therapy with systematic (i.e. before occurrence of a relapse) RTX retreatment was associated with longer relapse-free survival (41 versus 21 months; P = 0.02). Eight severe infections occurred in 4 patients during follow-up (severe infections rate of 12.1/100 patient-years) and hypogammaglobulinemia ≤5 g/l in 3 patients.
Conclusion: RTX is effective for both induction therapy and treatment of relapses in IgG4-RD, but relapses are frequent after B-cell reconstitution. Maintenance therapy with systematic RTX infusions is associated with longer relapse-free survival and might represent a novel treatment strategy. Yet, the high rate of infections and the temporary effect of RTX might be hindrances to such strategy.
Conflict of interest statement
Figures



References
-
- Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–92. doi: 10.1038/modpathol.2012.72 - DOI - PubMed
-
- Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366(6):539–51. doi: 10.1056/NEJMra1104650 - DOI - PubMed
-
- Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385(9976):1460–71. doi: 10.1016/S0140-6736(14)60720-0 - DOI - PubMed
-
- Ebbo M, Daniel L, Pavic M, Sève P, Hamidou M, Andres E, et al. IgG4-related systemic disease: features and treatment response in a French cohort: results of a multicenter registry. Medicine (Baltimore). 2012;91(1):49–56. - PubMed
-
- Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40(3):352–8. doi: 10.1097/MPA.0b013e3182142fd2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

