Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea
- PMID: 28915323
- PMCID: PMC5658794
- DOI: 10.1002/cphy.c160049
Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea
Abstract
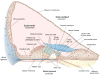
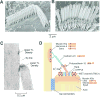
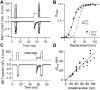
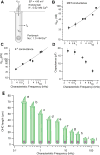
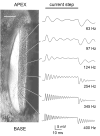
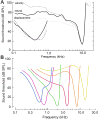
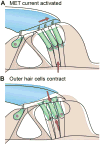
Sound pressure fluctuations striking the ear are conveyed to the cochlea, where they vibrate the basilar membrane on which sit hair cells, the mechanoreceptors of the inner ear. Recordings of hair cell electrical responses have shown that they transduce sound via submicrometer deflections of their hair bundles, which are arrays of interconnected stereocilia containing the mechanoelectrical transducer (MET) channels. MET channels are activated by tension in extracellular tip links bridging adjacent stereocilia, and they can respond within microseconds to nanometer displacements of the bundle, facilitated by multiple processes of Ca2+-dependent adaptation. Studies of mouse mutants have produced much detail about the molecular organization of the stereocilia, the tip links and their attachment sites, and the MET channels localized to the lower end of each tip link. The mammalian cochlea contains two categories of hair cells. Inner hair cells relay acoustic information via multiple ribbon synapses that transmit rapidly without rundown. Outer hair cells are important for amplifying sound-evoked vibrations. The amplification mechanism primarily involves contractions of the outer hair cells, which are driven by changes in membrane potential and mediated by prestin, a motor protein in the outer hair cell lateral membrane. Different sound frequencies are separated along the cochlea, with each hair cell being tuned to a narrow frequency range; amplification sharpens the frequency resolution and augments sensitivity 100-fold around the cell's characteristic frequency. Genetic mutations and environmental factors such as acoustic overstimulation cause hearing loss through irreversible damage to the hair cells or degeneration of inner hair cell synapses. © 2017 American Physiological Society. Compr Physiol 7:1197-1227, 2017.
Copyright © 2017 John Wiley & Sons, Inc.
Figures



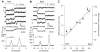


References
-
- Occupational Noise Exposure Cincinnati. OH: DHHS (NIOSH); 1998. pp. 2–3. Publication No. 98–126.
-
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–7034. - PMC - PubMed
-
- Akinpelu OV, Peleva E, Funnell WR, Daniel SJ. Otoacoustic emissions in newborn hearing screening: a systematic review of the effects of different protocols on test outcomes. Int J Pediatr Otorhinolaryngol. 2014;78:711–717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

