Hyperosmotic stress stimulates autophagy via polycystin-2
- PMID: 28915568
- PMCID: PMC5593539
- DOI: 10.18632/oncotarget.18995
Hyperosmotic stress stimulates autophagy via polycystin-2
Abstract
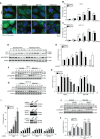
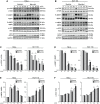
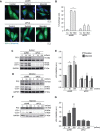
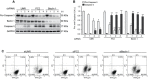
Various intracellular mechanisms are activated in response to stress, leading to adaptation or death. Autophagy, an intracellular process that promotes lysosomal degradation of proteins, is an adaptive response to several types of stress. Osmotic stress occurs under both physiological and pathological conditions, provoking mechanical stress and activating various osmoadaptive mechanisms. Polycystin-2 (PC2), a membrane protein of the polycystin family, is a mechanical sensor capable of activating the cell signaling pathways required for cell adaptation and survival. Here we show that hyperosmotic stress provoked by treatment with hyperosmolar concentrations of sorbitol or mannitol induces autophagy in HeLa and HCT116 cell lines. In addition, we show that mTOR and AMPK, two stress sensor proteins involved modulating autophagy, are downregulated and upregulated, respectively, when cells are subjected to hyperosmotic stress. Finally, our findings show that PC2 is required to promote hyperosmotic stress-induced autophagy. Downregulation of PC2 prevents inhibition of hyperosmotic stress-induced mTOR pathway activation. In conclusion, our data provide new insight into the role of PC2 as a mechanosensor that modulates autophagy under hyperosmotic stress conditions.
Keywords: Autophagy; hyperosmotic stress; mTOR; polycystin-2.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that no competing interests exist.
Figures
References
-
- Lemp MA, Bron AJ, Baudouin C, Benitez Del Castillo JM, Geffen D, Tauber J, Foulks GN, Pepose JS, Sullivan BD. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792–8. e1. - PubMed
-
- Stookey JD. High prevalence of plasma hypertonicity among community-dwelling older adults: results from NHANES III. J Am Diet Assoc. 2005;105:1231–1239. - PubMed
-
- Stookey JD, Barclay D, Arieff A, Popkin BM. The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr. 2007;61:190–199. - PubMed
-
- Berga-Bolanos R, Drews-Elger K, Aramburu J, Lopez-Rodriguez C. NFAT5 regulates T lymphocyte homeostasis and CD24-dependent T cell expansion under pathologic hypernatremia. J Immunol. 2010;185:6624–6635. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

