Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha
- PMID: 28915577
- PMCID: PMC5593548
- DOI: 10.18632/oncotarget.18125
Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha
Abstract
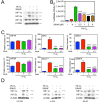
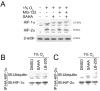
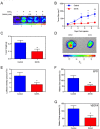
Histone deacetylase inhibitors (HDACis) are a potent class of tumor-suppressive agents traditionally believed to exert their effects through loosening tightly-wound chromatin resulting in de-inhibition of various tumor suppressive genes. Recent literature however has shown altered intratumoral hypoxia signaling with HDACi administration not attributable to changes in chromatin structure. We sought to determine the precise mechanism of HDACi-mediated hypoxia signaling attenuation using vorinostat (SAHA), an FDA-approved class I/IIb/IV HDACi. Through an in-vitro and in-vivo approach utilizing cell lines for hepatocellular carcinoma (HCC), osteosarcoma (OS), and glioblastoma (GBM), we demonstrate that SAHA potently inhibits HIF-a nuclear translocation via direct acetylation of its associated chaperone, heat shock protein 90 (Hsp90). In the presence of SAHA we found elevated levels of acetyl-Hsp90, decreased interaction between acetyl-Hsp90 and HIF-a, decreased nuclear/cytoplasmic HIF-α expression, absent HIF-α association with its nuclear karyopharyin Importin, and markedly decreased HIF-a transcriptional activity. These changes were associated with downregulation of downstream hypoxia molecules such as endothelin 1, erythropoietin, glucose transporter 1, and vascular endothelial growth factor. Findings were replicated in an in-vivo Hep3B HRE-Luc expressing xenograft, and were associated with significant decreases in xenograft tumor size. Altogether, this study highlights a novel mechanism of action of an important class of chemotherapeutic.
Keywords: HDACi; HIF; Hsp90; SAHA; hypoxia.
Conflict of interest statement
CONFLICTS OF INTEREST The authors disclose no potential conflicts of interest.
Figures




Similar articles
-
The histone deacetylase inhibitor, Vorinostat, represses hypoxia inducible factor 1 alpha expression through translational inhibition.PLoS One. 2014 Aug 28;9(8):e106224. doi: 10.1371/journal.pone.0106224. eCollection 2014. PLoS One. 2014. PMID: 25166596 Free PMC article.
-
Anticancer activity of MPT0G157, a derivative of indolylbenzenesulfonamide, inhibits tumor growth and angiogenesis.Oncotarget. 2015 Jul 30;6(21):18590-601. doi: 10.18632/oncotarget.4068. Oncotarget. 2015. PMID: 26087180 Free PMC article.
-
Immunoexpression patterns for Hypoxia-inducible Factor-1α and von Hippel-Lindau protein, in relation to Hsp90, of human brain tumors.Histol Histopathol. 2016 May;31(5):535-46. doi: 10.14670/HH-11-692. Epub 2015 Nov 23. Histol Histopathol. 2016. PMID: 26592496
-
Hypoxic "second hit" in leukocytes from trauma patients: Modulation of the immune response by histone deacetylase inhibition.Cytokine. 2010 Mar;49(3):303-11. doi: 10.1016/j.cyto.2009.11.013. Epub 2009 Dec 28. Cytokine. 2010. PMID: 20056553
-
Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1alpha induction in response to hypoxic stress and growth factors.Cancer Res. 2005 Jun 1;65(11):4918-28. doi: 10.1158/0008-5472.CAN-04-4453. Cancer Res. 2005. PMID: 15930314
Cited by
-
CCL5/CCR5 axis in human diseases and related treatments.Genes Dis. 2022 Jan;9(1):12-27. doi: 10.1016/j.gendis.2021.08.004. Epub 2021 Aug 26. Genes Dis. 2022. PMID: 34514075 Free PMC article. Review.
-
Therapeutic Drug-Induced Metabolic Reprogramming in Glioblastoma.Cells. 2022 Sep 22;11(19):2956. doi: 10.3390/cells11192956. Cells. 2022. PMID: 36230918 Free PMC article. Review.
-
Vorinostat in autophagic cell death: A critical insight into autophagy-mediated, -associated and -dependent cell death for cancer prevention.Drug Discov Today. 2022 Jan;27(1):269-279. doi: 10.1016/j.drudis.2021.08.004. Epub 2021 Aug 13. Drug Discov Today. 2022. PMID: 34400351 Free PMC article. Review.
-
Hypoxia-inducible transcription factors: architects of tumorigenesis and targets for anticancer drug discovery.Transcription. 2025 Feb;16(1):86-117. doi: 10.1080/21541264.2024.2417475. Epub 2024 Oct 29. Transcription. 2025. PMID: 39470609 Free PMC article. Review.
-
Proteostasis Modulation in Germline Missense von Hippel Lindau Disease.Clin Cancer Res. 2023 Jun 13;29(12):2199-2209. doi: 10.1158/1078-0432.CCR-22-3651. Clin Cancer Res. 2023. PMID: 37018064 Free PMC article.
References
-
- Warfel NA, El-Deiry WS. HIF-1 signaling in drug resistance to chemotherapy. Curr Med Chem. 2014;21:3021–8. - PubMed
-
- Doublier S, Belisario DC, Polimeni M, Annaratone L, Riganti C, Allia E, Ghigo D, Bosia A, Sapino A. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12:4. doi: 10.1186/1471-2407-12-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

