Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer
- PMID: 28915623
- PMCID: PMC5593594
- DOI: 10.18632/oncotarget.18082
Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer
Abstract
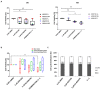
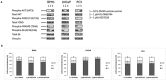
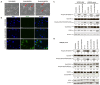
The PI3K/AKT/mTOR pathway is frequently activated in advanced prostate cancer, due to loss of the tumour suppressor PTEN, and is an important axis for drug development. We have assessed the molecular and functional consequences of pathway blockade by inhibiting AKT and mTOR kinases either in combination or as individual drug treatments. In established prostate cancer cell lines, a decrease in cell viability and in phospho-biomarker expression was observed. Although apoptosis was not induced, a G1 growth arrest was observed in PTEN null LNCaP cells, but not in BPH1 or PC3 cells. In contrast, when the AKT inhibitor AZD7328 was applied to patient-derived prostate cultures that retained expression of PTEN, activation of a compensatory Ras/MEK/ERK pathway was observed. Moreover, whilst autophagy was induced following treatment with AZD7328, cell viability was less affected in the patient-derived cultures than in cell lines. Surprisingly, treatment with a combination of both AZD7328 and two separate MEK1/2 inhibitors further enhanced phosphorylation of ERK1/2 in primary prostate cultures. However, it also induced irreversible growth arrest and senescence. Ex vivo treatment of a patient-derived xenograft (PDX) of prostate cancer with a combination of AZD7328 and the mTOR inhibitor KU-0063794, significantly reduced tumour frequency upon re-engraftment of tumour cells. The results demonstrate that single agent targeting of the PI3K/AKT/mTOR pathway triggers activation of the Ras/MEK/ERK compensatory pathway in near-patient samples. Therefore, blockade of one pathway is insufficient to treat prostate cancer in man.
Keywords: AKT; MAPK signalling; RAS; autophagy; mTOR; prostate cancer.
Conflict of interest statement
CONFLICTS OF INTEREST Barry Davies is an employee and shareholder of AstraZeneca. No potential conflicts of interest was disclosed by the other authors.
Figures

Similar articles
-
Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance.Adv Enzyme Regul. 2006;46:249-79. doi: 10.1016/j.advenzreg.2006.01.004. Epub 2006 Jul 18. Adv Enzyme Regul. 2006. PMID: 16854453
-
Synthetic lethal interaction between PI3K/Akt/mTOR and Ras/MEK/ERK pathway inhibition in rhabdomyosarcoma.Cancer Lett. 2013 Sep 1;337(2):200-9. doi: 10.1016/j.canlet.2013.05.010. Epub 2013 May 16. Cancer Lett. 2013. PMID: 23684925
-
Dual blockade of PI3K/AKT/mTOR (NVP-BEZ235) and Ras/Raf/MEK (AZD6244) pathways synergistically inhibit growth of primary endometrioid endometrial carcinoma cultures, whereas NVP-BEZ235 reduces tumor growth in the corresponding xenograft models.Gynecol Oncol. 2015 Jul;138(1):165-73. doi: 10.1016/j.ygyno.2015.04.028. Epub 2015 Apr 28. Gynecol Oncol. 2015. PMID: 25933683
-
Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy.Mol Biomed. 2022 Dec 21;3(1):47. doi: 10.1186/s43556-022-00110-2. Mol Biomed. 2022. PMID: 36539659 Free PMC article. Review.
-
Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia.Leukemia. 2008 Apr;22(4):686-707. doi: 10.1038/leu.2008.26. Epub 2008 Mar 13. Leukemia. 2008. PMID: 18337767 Review.
Cited by
-
Gain-of-Function RHOA Mutations Promote Focal Adhesion Kinase Activation and Dependency in Diffuse Gastric Cancer.Cancer Discov. 2020 Feb;10(2):288-305. doi: 10.1158/2159-8290.CD-19-0811. Epub 2019 Nov 26. Cancer Discov. 2020. PMID: 31771969 Free PMC article.
-
Rab7‑mediated autophagy regulates phenotypic transformation and behavior of smooth muscle cells via the Ras/Raf/MEK/ERK signaling pathway in human aortic dissection.Mol Med Rep. 2019 Apr;19(4):3105-3113. doi: 10.3892/mmr.2019.9955. Epub 2019 Feb 14. Mol Med Rep. 2019. PMID: 30816458 Free PMC article.
-
MEK inhibition overcomes everolimus resistance in gastric cancer.Cancer Chemother Pharmacol. 2020 Jun;85(6):1079-1087. doi: 10.1007/s00280-020-04078-0. Epub 2020 May 22. Cancer Chemother Pharmacol. 2020. PMID: 32444897
-
Phospholipase D inhibitors reduce human prostate cancer cell proliferation and colony formation.Br J Cancer. 2018 Jan;118(2):189-199. doi: 10.1038/bjc.2017.391. Epub 2017 Nov 14. Br J Cancer. 2018. PMID: 29136407 Free PMC article.
-
Co-targeting PI3K, mTOR, and IGF1R with small molecule inhibitors for treating undifferentiated pleomorphic sarcoma.Cancer Biol Ther. 2017 Oct 3;18(10):816-826. doi: 10.1080/15384047.2017.1373230. Epub 2017 Nov 3. Cancer Biol Ther. 2017. PMID: 29099264 Free PMC article.
References
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. - PubMed
-
- Esch L, Schulz WA, Albers P. Sequential treatment with taxanes and novel anti-androgenic compounds in castration-resistant prostate cancer. Oncol Res Treat. 2014;37:492–498. - PubMed
-
- Beltran H, Beer TM, Carducci MA, de Bono J, Gleave M, Hussain M, Kelly WK, Saad F, Sternberg C, Tagawa ST, Tannock IF. New therapies for castration-resistant prostate cancer: efficacy and safety. Eur Urol. 2011;60:279–290. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

