Selection of optimal molecular targets for tumor-specific imaging in pancreatic ductal adenocarcinoma
- PMID: 28915633
- PMCID: PMC5593604
- DOI: 10.18632/oncotarget.18232
Selection of optimal molecular targets for tumor-specific imaging in pancreatic ductal adenocarcinoma
Abstract
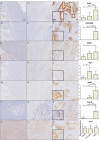
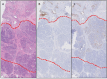
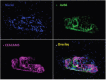
Discrimination of pancreatic ductal adenocarcinoma (PDAC) from chronic pancreatitis (CP) or peritumoral inflammation is challenging, both at preoperative imaging and during surgery, but it is crucial for proper therapy selection. Tumor-specific molecular imaging aims to enhance this discrimination and to help select and stratify patients for resection. We evaluated various biomarkers for the specific identification of PDAC and associated lymph node metastases. Using immunohistochemistry (IHC), expression levels and patterns were investigated of integrin αvβ6, carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), Cathepsin E (Cath E), epidermal growth factor receptor (EGFR), hepatocyte growth factor receptor (c-MET), thymocyte differentiation antigen 1 (Thy1), and urokinase-type plasminogen activator receptor (uPAR). In a first cohort, multiple types of pancreatic tissue were evaluated (n=62); normal pancreatic tissue (n=8), CP (n=7), PDAC (n=9), tumor associated lymph nodes (n=32), and PDAC after neoadjuvant radiochemotherapy (n=6). In a second cohort, tissues were investigated (n=55) with IHC and immunofluorescence (IF) for concordance of biomarker expression in all tissue types, obtained from an individual patient. Integrin αvβ6 and CEACAM5 showed significantly higher expression levels in PDAC versus normal pancreatic tissue (P=0.001 and P<0.001, respectively) and CP (P=0.003 and P<0.001, respectively). Avβ6 and CEACAM5 expression identified tumor-positive lymph nodes correctly in 84% and 68%, respectively, and in 100% of tumor-negative nodes for both biomarkers. In conclusion, αvβ6 and CEACAM5 are excellent biomarkers to differentiate PDAC from surrounding tissue and to identify lymph node metastases. Individually or combined, these biomarkers are promising targets for tumor-specific molecular imaging of PDAC.
Keywords: biomarker; molecular imaging; pancreatic cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

