Morphogenesis along the animal-vegetal axis: fates of primary quartet micromere daughters in the gastropod Crepidula fornicata
- PMID: 28915788
- PMCID: PMC5603038
- DOI: 10.1186/s12862-017-1057-1
Morphogenesis along the animal-vegetal axis: fates of primary quartet micromere daughters in the gastropod Crepidula fornicata
Abstract
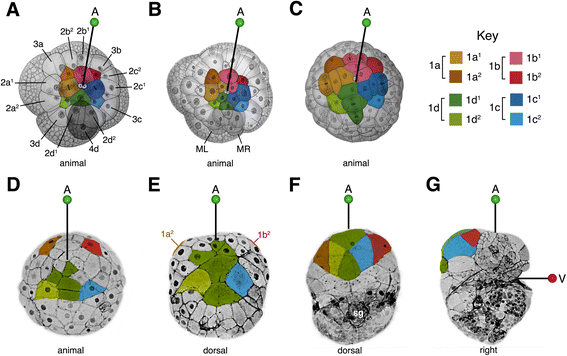
Background: The Spiralia are a large, morphologically diverse group of protostomes (e.g. molluscs, annelids, nemerteans) that share a homologous mode of early development called spiral cleavage. One of the most highly-conserved features of spiralian development is the contribution of the primary quartet cells, 1a-1d, to the anterior region of the embryo (including the brain, eyes, and the anterior ciliary band, called the prototroch). Yet, very few studies have analyzed the ultimate fates of primary quartet sub-lineages, or examined the morphogenetic events that take place in the anterior region of the embryo.
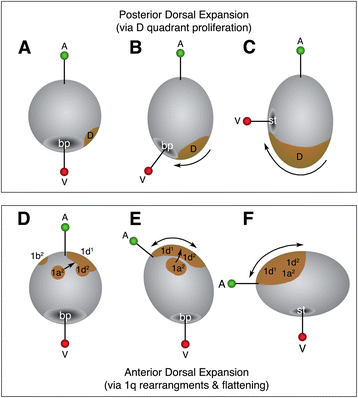
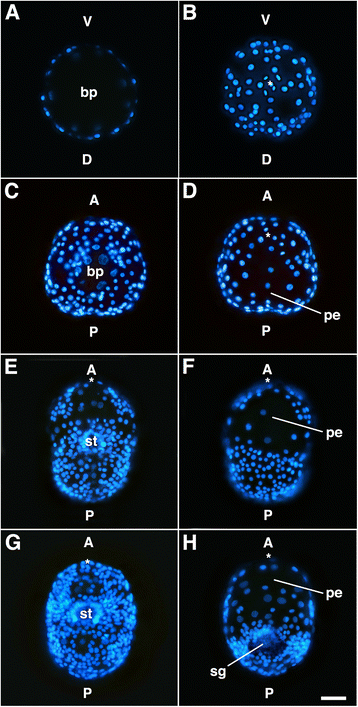
Results: This study focuses on the caenogastropod slipper snail, Crepidula fornicata, a model for molluscan developmental biology. Through direct lineage tracing of primary quartet daughter cells, and examination of these cells during gastrulation and organogenesis stages, we uncovered behaviors never described before in a spiralian. For the first time, we show that the 1a2-1d2 cells do not contribute to the prototroch (as they do in other species) and are ultimately lost before hatching. During gastrulation and anterior-posterior axial elongation stages, these cells cleavage-arrest and spread dramatically, contributing to a thin provisional epidermis on the dorsal side of the embryo. This spreading is coupled with the displacement of the animal pole, and other pretrochal cells, closer to the ventrally-positioned mouth, and the vegetal pole.
Conclusions: This is the first study to document the behavior and fate of primary quartet sub-lineages among molluscs. We speculate that the function of 1a2-1d2 cells (in addition to two cells derived from 1d12, and the 2b lineage) is to serve as a provisional epithelium that allows for anterior displacement of the other progeny of the primary quartet towards the anterior-ventral side of the embryo. These data support a new and novel mechanism for axial bending, distinct from canonical models in which axial bending is suggested to be driven primarily by differential proliferation of posterior dorsal cells. These data suggest also that examining sub-lineages in other spiralians will reveal greater variation than previously assumed.
Keywords: Axial elongation; Gastropoda; Lophotrochozoa; Mollusca; Morphogenesis; Prototroch; Spiralia; Trochoblasts.
Conflict of interest statement
Ethics approval and consent to participate
This study used wild-caught invertebrate snails and those adult animals were all returned back to the ocean after their embryos were collected.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

