MicroRNA-488 and -920 regulate the production of proinflammatory cytokines in acute gouty arthritis
- PMID: 28915828
- PMCID: PMC5602958
- DOI: 10.1186/s13075-017-1418-6
MicroRNA-488 and -920 regulate the production of proinflammatory cytokines in acute gouty arthritis
Abstract
Background: Gout is considered one of the most painful acute conditions caused by deposition of monosodium urate (MSU) crystals within joints. Recent studies have shown that interleukin (IL)-1β is a key inflammatory mediator in acute gouty arthritis (GA), and its level is regulated by microRNAs (miRNAs). However, the molecular mechanisms of the regulation remain unclear.
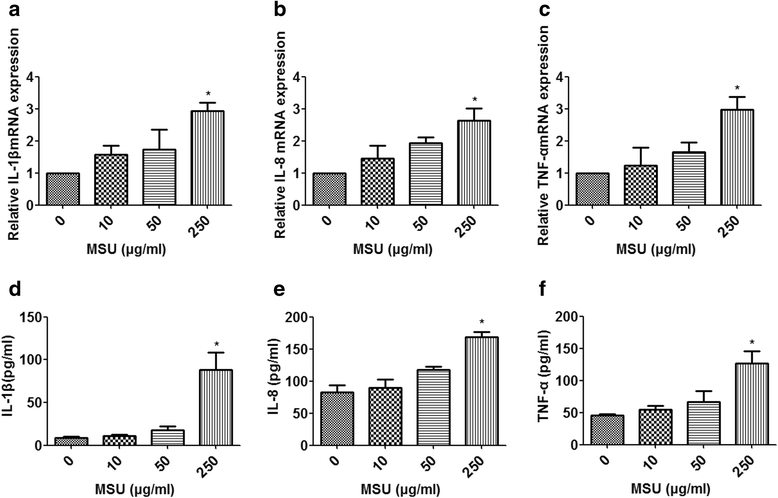
Methods: A miRNA microarray was used to analyze the miRNA expression profiles in peripheral white blood cells (WBCs) of patients with GA. THP-1 cells were transfected with miRNA mimics, stimulated by MSU crystals, and then subjected to quantitative real-time polymerase chain reaction or Western blot analysis. Levels of IL-1β, IL-8, and tumor necrosis factor (TNF)-α in culture supernatants of THP-1 cells were measured by enzyme-linked immunosorbent assay. A luciferase reporter assay was conducted to confirm the interaction of miRNA and IL-1β 3'-untranslated regions (UTRs).
Results: Combining bioinformatics and miRNA expression profiles, we found five miRNAs (hsa-miR-30c-1-3p, hsa-miR-488-3p, hsa-miR-550a-3p, hsa-miR-663a, and hsa-miR-920) that possibly target IL-1β. Then, we demonstrated that miR-488 and miR-920 were significantly decreased in the WBCs of patients with GA and that MSU crystals could inhibit expression of miR-488 and miR-920. Upregulation of miR-488 and miR-920 could suppress MSU-induced IL-1β protein expression in THP-1 cells, but no significant difference in IL-1β messenger RNA levels was observed. Moreover, we found that miR-488 and miR-920 could directly target the 3'-UTR of IL-1β. Overexpression of miR-488 and miR-920 could significantly inhibit the gene and protein expression of IL-8 and TNF-α in MSU-induced THP-1 cells.
Conclusions: This study demonstrates the roles of miR-488 and miR-920 in regulating the production of proinflammatory cytokines in the pathogenesis of GA. These findings suggest that miR-488 and miR-920 could serve as potential therapeutic targets in the treatment of GA.
Keywords: Gouty arthritis; Interleukin-1β; MicroRNA; Monosodium urate crystals; Proinflammatory cytokines.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xiamen University. The need for informed consent from the patients was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

