Assessment of consent models as an ethical consideration in the conduct of prehospital ambulance randomised controlled clinical trials: a systematic review
- PMID: 28915851
- PMCID: PMC5603026
- DOI: 10.1186/s12874-017-0423-4
Assessment of consent models as an ethical consideration in the conduct of prehospital ambulance randomised controlled clinical trials: a systematic review
Abstract
Background: We sought to understand the main ethical considerations when conducting clinical trials in the prehospital ambulance based setting.
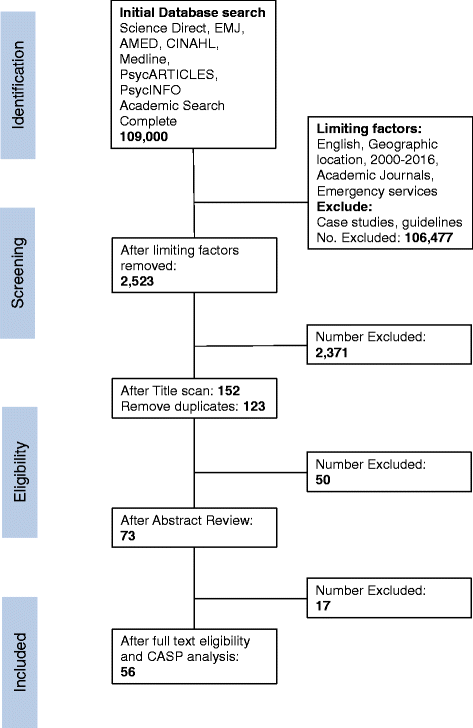
Methods: A systematic review of the literature on randomised controlled trials in ambulance settings was undertaken. A search of eight databases identified published studies involving recruitment of ambulance service users. Four independent authors undertook abstract and full-text reviews to determine eligibility and extract relevant data. The data extraction concentrated on ethical considerations, with any discussion of ethics being included for further analysis. The resultant data were combined to form a narrative synthesis.
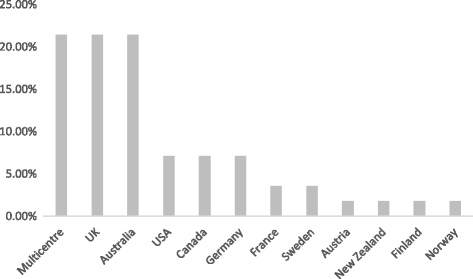
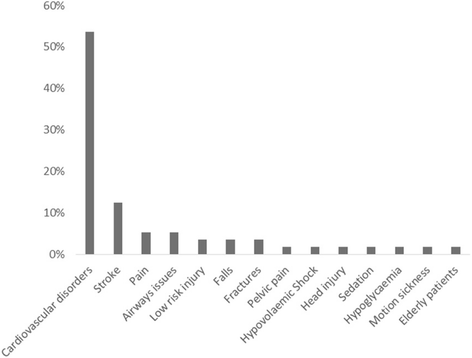
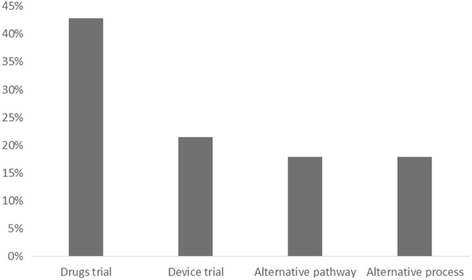
Results: In all, 56 papers were identified as meeting the inclusion criteria. Issues relating to consent were the most significant theme identified. Type of consent differed depending on the condition or intervention being studied. The country in which the research took place did not appear to influence the type of consent, apart from the USA where exception from consent appeared to be most commonly used. A wide range of terms were used to describe consent.
Conclusions: Consent was the main ethical consideration in published ambulance based research. A range of consent models were used ranging from informed consent to exception from consent (waiver of consent). Many studies cited international guidelines as informing their choice of consent model but diverse and sometimes confused terms were used to describe these models. This suggests that standardisation of consent models and the terminology used to describe them is warranted.
Keywords: Ambulance; Clinical trials; Consent; Ethics; Prehospital.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JPA is funded by the British Heart Foundation (BHF, CS/14/4/30972) and National Institute of Health Research (NIHR) Health Technology Assessment Programme (10/104/24). PB is Stroke Association Professor or Stroke Medicine, and is an NIHR Senior Investigator.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Gray JD. The problem of consent in emergency medicine research. Can J Emer Med. 2001;3(3):213–218. - PubMed
-
- Chin TL, Moore EE, Coors ME, Chandler JG, Ghasabyan A, Harr JN, Stringham JR, Ramos CR, Ammons S, Banerjee A, et al. Exploring ethical conflicts in emergency trauma research: the COMBAT (control of major bleeding after trauma) study experience. Surgery. 2015;157(1):10–19. doi: 10.1016/j.surg.2014.05.021. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical