TIGA-CUB - manualised psychoanalytic child psychotherapy versus treatment as usual for children aged 5-11 years with treatment-resistant conduct disorders and their primary carers: study protocol for a randomised controlled feasibility trial
- PMID: 28915904
- PMCID: PMC5602865
- DOI: 10.1186/s13063-017-2166-2
TIGA-CUB - manualised psychoanalytic child psychotherapy versus treatment as usual for children aged 5-11 years with treatment-resistant conduct disorders and their primary carers: study protocol for a randomised controlled feasibility trial
Abstract
Background: The National Institute for Health and Care Excellence (NICE) recommends evidence-based parenting programmes as a first-line intervention for conduct disorders (CD) in children aged 5-11 years. As these are not effective in 25-33% of cases, NICE has requested research into second-line interventions. Child and Adolescent Psychotherapists (CAPTs) address highly complex problems where first-line treatments have failed and there have been small-scale studies of Psychoanalytic Child Psychotherapy (PCP) for CD. A feasibility trial is needed to determine whether a confirmatory trial of manualised PCP (mPCP) versus Treatment as Usual (TaU) for CD is practicable or needs refinement. The aim of this paper is to publish the abridged protocol of this feasibility trial.
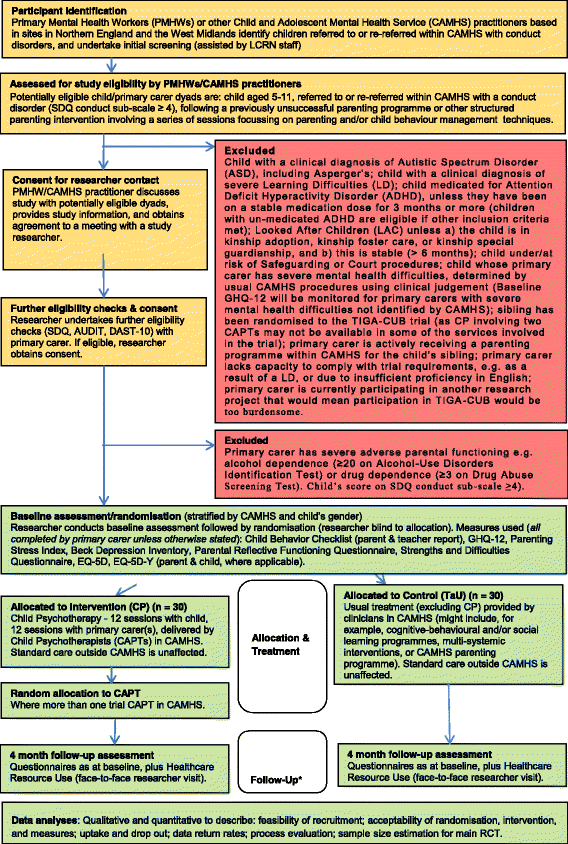
Methods and design: TIGA-CUB (Trial on improving Inter-Generational Attachment for Children Undergoing Behaviour problems) is a two-arm, pragmatic, parallel-group, multicentre, individually randomised (1:1) controlled feasibility trial (target n = 60) with blinded outcome assessment (at 4 and 8 months), which aims to develop an optimum practicable protocol for a confirmatory, pragmatic, randomised controlled trial (RCT) (primary outcome: child's behaviour; secondary outcomes: parental reflective functioning and mental health, child and parent quality of life), comparing mPCP and TaU as second-line treatments for children aged 5-11 years with treatment-resistant CD and inter-generational attachment difficulties, and for their primary carers. Child-primary carer dyads will be recruited following a referral to, or re-referral within, National Health Service (NHS) Child and Adolescent Mental Health Services (CAMHS) after an unsuccessful first-line parenting intervention. PCP will be delivered by qualified CAPTs working in routine NHS clinical practice, using a trial-specific PCP manual (a brief version of established PCP clinical practice). Outcomes are: (1) feasibility of recruitment methods, (2) uptake and follow-up rates, (3) therapeutic delivery, treatment retention and attendance, intervention adherence rates, (4) follow-up data collection, and (5) statistical, health economics, process evaluation, and qualitative outcomes.
Discussion: TIGA-CUB will provide important information on the feasibility and potential challenges of undertaking a confirmatory RCT to evaluate the effectiveness and cost-effectiveness of mPCP.
Trial registration: Current Controlled Trials, ID: ISRCTN86725795 . Registered on 31 May 2016.
Keywords: Conduct disorders; Inter-generational attachment; Psychoanalytic child psychotherapy; Randomised controlled trial; Treatment-resistant.
Conflict of interest statement
Authors’ information
EE is a research fellow at COMIC (Child Oriented Mental health Interventions Centre), York, part of Leeds and York Partnership NHS Foundation Trust, Child and Adolescent Psychotherapist at Banbury CAMHS, Oxford Health NHS Foundation Trust, research tutor and member of the Child Attachment and Psychological Therapies Research Unit (ChAPTRe) at UCL/the Anna Freud National Centre for Children and Families, and TIGA-CUB chief investigator.
RW is the Principal Statistician at the Clinical Trials Research Unit within the Leeds Institute of Clinical Trials Research, at the School of Medicine, University of Leeds, and supervising statistician.
KB is the Senior Trial Manager at the Clinical Trials Research Unit within the Leeds Institute of Clinical Trials Research, at the School of Medicine, University of Leeds.
RC is a Medical Statistician at the Clinical Trials Research Unit within the Leeds Institute of Clinical Trials Research, at the School of Medicine, University of Leeds, and former trial statistician.
LG is based at the Clinical Trials Research Unit within the Leeds Institute of Clinical Trials Research, at the School of Medicine, University of Leeds, with expertise in the delivery of complex intervention trials.
SR is Senior Trial Manager at the Clinical Trials Research Unit within the Leeds Institute of Clinical Trials Research, at the School of Medicine, University Leeds, and is the trial manager.
ST is an Associate Professor in Health Economics at the Academic Unit of Health Economics, Leeds Institute of Health Sciences, School of Medicine, University of Leeds.
MT is a Senior Research Fellow at the Centre for Health Services Research, Leeds Institute of Health Sciences, School of Medicine, University of Leeds.
AWH is a Senior Medical Statistician at the Clinical Trials Research Unit within the Leeds Institute of Clinical Trials Research, at the School of Medicine, University of Leeds, and is the trial statistician.
LE is the Lead Director at the Northern School of Child and Adolescent Psychotherapy based in Leeds and hosted by Leeds and York Partnership Foundation NHS Trust, Consultant Child and Adolescent Psychotherapist and Psychoanalyst (British Psychoanalytical Society).
DE is the Head of Children’s Social Work Services at City of York Council.
TH is a Consultant General Adult Psychiatrist and Senior Lecturer in Psychiatry, University of Leeds.
NM is a Senior Lecturer in the Research Department of Clinical, Educational and Health Psychology at UCL, and Co-Director of the Child Attachment and Psychological Therapies Research Unit (ChAPTRe), at UCL/the Anna Freud National Centre for Children and Families.
PW is a Consultant Child and Adolescent Clinical Psychologist at the Winnicott Centre and the Director of Psychological Services, CAMHS, Central Manchester University Hospitals NHS Foundation Trust.
DC is a Professor of Child and Adolescent Psychiatry at the Leeds Institute of Health Sciences, School of Medicine, University of Leeds.
Ethics approval and consent to participate
Ethical approval was granted for the trial by Yorkshire and The Humber Bradford Leeds Research Ethics Committee (Reference: 16/YH/0055). The right of participants to refuse participation without giving reasons will be respected and participants will remain free to withdraw from the trial at any time without giving reasons and without prejudicing further treatment. Primary carers will make any such decision on behalf of the child.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Similar articles
-
TIGA-CUB-manualised psychoanalytic child psychotherapy versus treatment as usual for children aged 5-11 with treatment-resistant conduct disorders and their primary carers: results from a randomised controlled feasibility trial.J Child Adolesc Ment Health. 2018 Nov;30(3):167-182. doi: 10.2989/17280583.2018.1532433. Epub 2018 Nov 14. J Child Adolesc Ment Health. 2018. PMID: 30428772 Clinical Trial.
-
A modified video-feedback intervention for carers of foster children aged 6 years and under with reactive attachment disorder: a feasibility study and pilot RCT.Health Technol Assess. 2022 Aug;26(35):1-106. doi: 10.3310/SLIZ1119. Health Technol Assess. 2022. PMID: 35959710 Free PMC article. Clinical Trial.
-
A pragmatic randomised controlled trial and economic evaluation of family therapy versus treatment as usual for young people seen after second or subsequent episodes of self-harm: the Self-Harm Intervention - Family Therapy (SHIFT) trial.Health Technol Assess. 2018 Mar;22(12):1-222. doi: 10.3310/hta22120. Health Technol Assess. 2018. PMID: 29532784 Free PMC article. Clinical Trial.
-
Using routinely recorded data in the UK to assess outcomes in a randomised controlled trial: The Trials of Access.Trials. 2017 Aug 23;18(1):389. doi: 10.1186/s13063-017-2135-9. Trials. 2017. PMID: 28835254 Free PMC article. Review.
-
Therapeutic Interventions for Trauma-exposed Infants, Young Children, and Their Caregivers.Child Adolesc Psychiatr Clin N Am. 2025 Apr;34(2):291-310. doi: 10.1016/j.chc.2024.08.005. Epub 2024 Oct 3. Child Adolesc Psychiatr Clin N Am. 2025. PMID: 40044268 Review.
Cited by
-
Measuring health-related quality of life measures in children: lessons from a pilot study.Res Psychother. 2022 May 9;25(1):581. doi: 10.4081/ripppo.2022.581. Res Psychother. 2022. PMID: 35532026 Free PMC article.
References
-
- NICE . Antisocial behaviour and conduct disorders in children and young people: recognition, intervention and management (National Clinical Guideline 158). Commissioned by the National Institute for Health and Care Excellence. London: The British Psychological Society and The Royal College of Psychiatrists; 2013.
-
- Green H, et al. Mental health of children and young people in Great Britain, 2004. Basingstoke, Hampshire: Office for National Statistics and Palgrave McMillan; 2005.
-
- McCrone P, et al. Paying the price: the cost of mental health care in England to 2026. London: King’s Fund; 2008.
-
- Piquero A, et al. Trajectories of offending and their relation to life failure in late middle age: findings from the Cambridge Study in Delinquent Development. J Res Crime Delinquency. 2010;47(2):151–73. doi: 10.1177/0022427809357713. - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials