Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth
- PMID: 28916248
- PMCID: PMC5624555
- DOI: 10.1016/j.vaccine.2017.09.017
Breast cancer vaccines delivered by dendritic cell-targeted lentivectors induce potent antitumor immune responses and protect mice from mammary tumor growth
Abstract
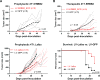
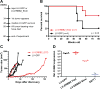
Breast cancer immunotherapy is a potent treatment option, with antibody therapies such as trastuzumab increasing 2-year survival rates by 50%. However, active immunotherapy through vaccination has generally been clinically ineffective. One potential means of improving vaccine therapy is by delivering breast cancer antigens to dendritic cells (DCs) for enhanced antigen presentation. To accomplish this in vivo, we pseudotyped lentiviral vector (LV) vaccines with a modified Sindbis Virus glycoprotein so that they could deliver genes encoding the breast cancer antigen alpha-lactalbumin (Lalba) or erb-b2 receptor tyrosine kinase 2 (ERBB2 or HER2) directly to resident DCs. We hypothesized that utilizing these DC-targeting lentiviral vectors asa breast cancer vaccine could lead to an improved immune response against self-antigens found in breast cancer tumors. Indeed, single injections of the vaccine vectors were able to amplify antigen-specific CD8T cells 4-6-fold over naïve mice, similar to the best published vaccine regimens. Immunization of these mice completely inhibited tumor growth in a foreign antigen environment (LV-ERBB2 in wildtype mice), and it reduced the rate of tumor growth in a self-antigen environment (LV-Lalba in wildtype or LV-ERBB2 in MMTV-huHER2 transgenic). These results show that a single injection with targeted lentiviral vectors can be an effective immunotherapy for breast cancer. Furthermore, they could be combined with other immunotherapeutic regimens to improve outcomes for patients with breast cancer.
Keywords: Alpha-lactalbumin; Breast cancer immunotherapy; Cancer vaccine; ERBB2/Her2; Lentiviral vector.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

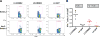
References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine. 2001;344(11):783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

