SIDT2 Transports Extracellular dsRNA into the Cytoplasm for Innate Immune Recognition
- PMID: 28916264
- PMCID: PMC5679266
- DOI: 10.1016/j.immuni.2017.08.007
SIDT2 Transports Extracellular dsRNA into the Cytoplasm for Innate Immune Recognition
Abstract
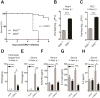
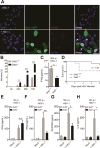
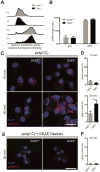
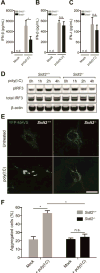
Double-stranded RNA (dsRNA) is a common by-product of viral infections and acts as a potent trigger of antiviral immunity. In the nematode C. elegans, sid-1 encodes a dsRNA transporter that is highly conserved throughout animal evolution, but the physiological role of SID-1 and its orthologs remains unclear. Here, we show that the mammalian SID-1 ortholog, SIDT2, is required to transport internalized extracellular dsRNA from endocytic compartments into the cytoplasm for immune activation. Sidt2-deficient mice exposed to extracellular dsRNA, encephalomyocarditis virus (EMCV), and herpes simplex virus 1 (HSV-1) show impaired production of antiviral cytokines and-in the case of EMCV and HSV-1-reduced survival. Thus, SIDT2 has retained the dsRNA transport activity of its C. elegans ortholog, and this transport is important for antiviral immunity.
Keywords: EMCV; HSV; MAVS; SIDT2; bystander immunity; double-stranded RNA; type I interferon; virus infection.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Aubrey BJ, Kelly GL, Kueh AJ, Brennan MS, O’Connor L, Milla L, Wilcox S, Tai L, Strasser A, Herold MJ. An inducible lentiviral guide RNA platform enables the identification of tumor-essential genes and tumor-promoting mutations in vivo. Cell Rep. 2015;10:1422–1432. - PubMed
-
- Barbosa-Morais NL, Irimia M, Pan Q, Xiong HY, Gueroussov S, Lee LJ, Slobodeniuc V, Kutter C, Watt S, Colak R, et al. The evolutionary landscape of alternative splicing in vertebrate species. Science. 2012;338:1587–1593. - PubMed
-
- Buschow SI, Lasonder E, Szklarczyk R, Oud MM, de Vries IJ, Figdor CG. Unraveling the human dendritic cell phagosome proteome by organellar enrichment ranking. Journal of proteomics. 2012;75:1547–1562. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

