AQ-13, an investigational antimalarial, versus artemether plus lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria: a randomised, phase 2, non-inferiority clinical trial
- PMID: 28916443
- PMCID: PMC5700806
- DOI: 10.1016/S1473-3099(17)30365-1
AQ-13, an investigational antimalarial, versus artemether plus lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria: a randomised, phase 2, non-inferiority clinical trial
Abstract
Background: Chloroquine was used for malaria treatment until resistant Plasmodium falciparum was identified. Because 4-aminoquinolines with modified side chains, such as AQ-13, are active against resistant parasites, we compared AQ-13 against artemether plus lumefantrine for treatment of uncomplicated P falciparum malaria.
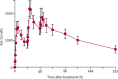
Methods: We did a randomised, non-inferiority trial. We screened men (≥18 years) with uncomplicated malaria in Missira (northeast Mali) and Bamako (capital of Mali) for eligibility (≥2000 asexual P falciparum parasites per μL of blood). Eligible participants were randomly assigned to either the artemether plus lumefantrine group or AQ-13 group by permuting blocks of four with a random number generator. Physicians and others caring for the participants were masked, except for participants who received treatment and the research pharmacist who implemented the randomisation and provided treatment. Participants received either 80 mg of oral artemether and 480 mg of oral lumefantrine twice daily for 3 days or 638·50 mg of AQ-13 base (two oral capsules) on days 1 and 2, and 319·25 mg base (one oral capsule) on day 3. Participants were monitored for parasite clearance (50 μL blood samples twice daily at 12 h intervals until two consecutive negative samples were obtained) and interviewed for adverse events (once every day) as inpatients during week 1. During the 5-week outpatient follow-up, participants were examined for adverse events and recurrent infection twice per week. All participants were included in the intention-to-treat analysis and per-protocol analysis, except for those who dropped out in the per-protocol analysis. The composite primary outcome was clearance of asexual parasites and fever by day 7, and absence of recrudescent infection by parasites with the same molecular markers from days 8 to 42 (defined as cure). Non-inferiority was considered established if the proportion of patients who were cured was higher for artemether plus lumefantrine than for AQ-13 and the upper limit of the 95% CI was less than the non-inferiority margin of 15%. This trial is registered at ClinicalTrials.gov, number NCT01614964.
Findings: Between Aug 6 and Nov 18, 2013, and between Sept 18 and Nov 20, 2015, 66 Malian men with uncomplicated malaria were enrolled. 33 participants were randomly assigned to each group. There were no serious adverse events (grade 2-4) and asexual parasites were cleared by day 7 in both groups. 453 less-severe adverse events (≤grade 1) were reported: 214 in the combination group and 239 in the AQ-13 group. Two participants withdrew from the AQ-13 group after parasite clearance and three were lost to follow-up. In the artemether plus lumefantrine group, two participants had late treatment failures (same markers as original isolates). On the basis of the per-protocol analysis, the AQ-13 and artemether plus lumefantrine groups had similar proportions cured (28 [100%] of 28 vs 31 [93·9%] of 33; p=0·50) and AQ-13 was not inferior to artemether plus lumefantrine (difference -6·1%, 95% CI -14·7 to 2·4). Proportions cured were also similar between the groups in the intention-to-treat analysis (28 of 33, 84·8% for AQ-13 vs 31 of 33, 93·9% for artemether and lumefantrine; p=0·43) but the upper bound of the 95% CI exceeded the 15% non-inferiority margin (difference 9·1%, 95% CI -5·6 to 23·8).
Interpretation: The per-protocol analysis suggested non-inferiority of AQ-13 to artemether plus lumefantrine. By contrast, the intention-to-treat analysis, which included two participants who withdrew and three who were lost to follow-up from the AQ-13 group, did not meet the criterion for non-inferiority of AQ-13, although there were no AQ-13 treatment failures. Studies with more participants (and non-immune participants) are needed to decide whether widespread use of modified 4-aminoquinolones should be recommended.
Funding: US Food and Drug Administration Orphan Product Development, National Institutes of Health, US Centers for Disease Control and Prevention, Burroughs-Wellcome Fund, US State Department, and WHO.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Treating malaria: new drugs for a new era.Lancet Infect Dis. 2017 Dec;17(12):1223-1224. doi: 10.1016/S1473-3099(17)30475-9. Epub 2017 Sep 12. Lancet Infect Dis. 2017. PMID: 28916444 No abstract available.
References
-
- Young MD, Contacos PG. Drug resistance in Plasmodium falciparum from Thailand. Am J Trop Med Hyg. 1963;12:305–314. - PubMed
-
- Comer RD, Young MD, Porter JA, Jr, Gauld JR, Merritt W. Chloroquine resistance in falciparum malaria on the Pacific coast of Colombia. Am J Trop Med Hyg. 1968;17:795–799. - PubMed
-
- Menard D, Matsika-Claquin MD, Djalle D. Association of failures of seven-day courses of artesunate in a non-immune population in Bangui, Central African Republic with decreased sensitivity of Plasmodium falciparum. Am J Trop Med Hyg. 2005;73:616–621. - PubMed
-
- Weniger BG, Blumberg RS, Campbell CC, Jones TC, Mount DL, Friedman SM. High-level chloroquine resistance of Plasmodium falciparum malaria acquired in Kenya. N Engl J Med. 1982;307:1560–1562. - PubMed
-
- Walker AJ, Lopez-Antunano FJ. Response to drugs of South American strains of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1968;62:654–657. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

