Behavioral, Modeling, and Electrophysiological Evidence for Supramodality in Human Metacognition
- PMID: 28916521
- PMCID: PMC6596112
- DOI: 10.1523/JNEUROSCI.0322-17.2017
Behavioral, Modeling, and Electrophysiological Evidence for Supramodality in Human Metacognition
Abstract
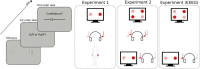
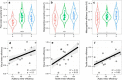
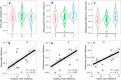
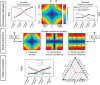
Human metacognition, or the capacity to introspect on one's own mental states, has been mostly characterized through confidence reports in visual tasks. A pressing question is to what extent results from visual studies generalize to other domains. Answering this question allows determining whether metacognition operates through shared, supramodal mechanisms or through idiosyncratic, modality-specific mechanisms. Here, we report three new lines of evidence for decisional and postdecisional mechanisms arguing for the supramodality of metacognition. First, metacognitive efficiency correlated among auditory, tactile, visual, and audiovisual tasks. Second, confidence in an audiovisual task was best modeled using supramodal formats based on integrated representations of auditory and visual signals. Third, confidence in correct responses involved similar electrophysiological markers for visual and audiovisual tasks that are associated with motor preparation preceding the perceptual judgment. We conclude that the supramodality of metacognition relies on supramodal confidence estimates and decisional signals that are shared across sensory modalities.SIGNIFICANCE STATEMENT Metacognitive monitoring is the capacity to access, report, and regulate one's own mental states. In perception, this allows rating our confidence in what we have seen, heard, or touched. Although metacognitive monitoring can operate on different cognitive domains, we ignore whether it involves a single supramodal mechanism common to multiple cognitive domains or modality-specific mechanisms idiosyncratic to each domain. Here, we bring evidence in favor of the supramodality hypothesis by showing that participants with high metacognitive performance in one modality are likely to perform well in other modalities. Based on computational modeling and electrophysiology, we propose that supramodality can be explained by the existence of supramodal confidence estimates and by the influence of decisional cues on confidence estimates.
Keywords: EEG; audiovisual; confidence; metacognition; signal detection theory; supramodality.
Copyright © 2018 the authors 0270-6474/18/380263-15$15.00/0.
Figures
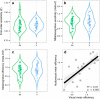
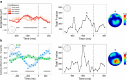
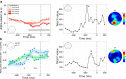
Similar articles
-
Metacognition across sensory modalities: Vision, warmth, and nociceptive pain.Cognition. 2019 May;186:32-41. doi: 10.1016/j.cognition.2019.01.018. Epub 2019 Feb 7. Cognition. 2019. PMID: 30739057 Free PMC article.
-
Correlated individual differences suggest a common mechanism underlying metacognition in visual perception and visual short-term memory.Proc Biol Sci. 2017 Nov 29;284(1867):20172035. doi: 10.1098/rspb.2017.2035. Proc Biol Sci. 2017. PMID: 29167365 Free PMC article.
-
Domain-specific and domain-general processes underlying metacognitive judgments.Conscious Cogn. 2017 Mar;49:264-277. doi: 10.1016/j.concog.2017.01.011. Epub 2017 Mar 8. Conscious Cogn. 2017. PMID: 28222381
-
Metacognition in Multisensory Perception.Trends Cogn Sci. 2016 Oct;20(10):736-747. doi: 10.1016/j.tics.2016.08.006. Epub 2016 Sep 6. Trends Cogn Sci. 2016. PMID: 27612983 Review.
-
Metacognition and Confidence: A Review and Synthesis.Annu Rev Psychol. 2024 Jan 18;75:241-268. doi: 10.1146/annurev-psych-022423-032425. Epub 2023 Sep 18. Annu Rev Psychol. 2024. PMID: 37722748 Review.
Cited by
-
Performance monitoring for sensorimotor confidence: A visuomotor tracking study.Cognition. 2020 Dec;205:104396. doi: 10.1016/j.cognition.2020.104396. Epub 2020 Aug 5. Cognition. 2020. PMID: 32771212 Free PMC article.
-
The folded X-pattern is not necessarily a statistical signature of decision confidence.PLoS Comput Biol. 2019 Oct 21;15(10):e1007456. doi: 10.1371/journal.pcbi.1007456. eCollection 2019 Oct. PLoS Comput Biol. 2019. PMID: 31634359 Free PMC article.
-
Consciousness is more than meets the eye: a call for a multisensory study of subjective experience.Neurosci Conscious. 2017 Mar 18;2017(1):nix003. doi: 10.1093/nc/nix003. eCollection 2017. Neurosci Conscious. 2017. PMID: 30042838 Free PMC article.
-
Thinking about thinking: A coordinate-based meta-analysis of neuroimaging studies of metacognitive judgements.Brain Neurosci Adv. 2018 Nov 13;2:2398212818810591. doi: 10.1177/2398212818810591. eCollection 2018. Brain Neurosci Adv. 2018. PMID: 30542659 Free PMC article. Review.
-
Domain-general enhancements of metacognitive ability through adaptive training.J Exp Psychol Gen. 2019 Jan;148(1):51-64. doi: 10.1037/xge0000505. J Exp Psychol Gen. 2019. PMID: 30596440 Free PMC article.
References
-
- Bates DM, Kliegl R, Vasishth S, Baayen H (2015) Parsimonious mixed models. arXiv preprint arXiv:150 604967:1–27.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
