CmeABC Multidrug Efflux Pump Contributes to Antibiotic Resistance and Promotes Campylobacter jejuni Survival and Multiplication in Acanthamoeba polyphaga
- PMID: 28916560
- PMCID: PMC5666138
- DOI: 10.1128/AEM.01600-17
CmeABC Multidrug Efflux Pump Contributes to Antibiotic Resistance and Promotes Campylobacter jejuni Survival and Multiplication in Acanthamoeba polyphaga
Abstract
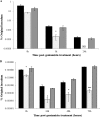
Campylobacter jejuni is a foodborne pathogen that is recognized as the leading cause of human bacterial gastroenteritis. The widespread use of antibiotics in medicine and in animal husbandry has led to an increased incidence of antibiotic resistance in Campylobacter In addition to a role in multidrug resistance (MDR), the Campylobacter CmeABC resistance-nodulation-division (RND)-type efflux pump may be involved in virulence. As a vehicle for pathogenic microorganisms, the protozoan Acanthamoeba is a good model for investigations of bacterial survival in the environment and the molecular mechanisms of pathogenicity. The interaction between C. jejuni 81-176 and Acanthamoeba polyphaga was investigated in this study by using a modified gentamicin protection assay. In addition, a possible role for the CmeABC MDR pump in this interaction was explored. Here we report that this MDR pump is beneficial for the intracellular survival and multiplication of C. jejuni in A. polyphaga but is dispensable for biofilm formation and motility.IMPORTANCE The endosymbiotic relationship between amoebae and microbial pathogens may contribute to persistence and spreading of the latter in the environment, which has significant implications for human health. In this study, we found that Campylobacter jejuni was able to survive and to multiply inside Acanthamoeba polyphaga; since these microorganisms can coexist in the same environment (e.g., on poultry farms), the latter may increase the risk of infection with Campylobacter Our data suggest that, in addition to its role in antibiotic resistance, the CmeABC MDR efflux pump plays a role in bacterial survival within amoebae. Furthermore, we demonstrated synergistic effects of the CmeABC MDR efflux pump and TetO on bacterial resistance to tetracycline. Due to its role in both the antibiotic resistance and the virulence of C. jejuni, the CmeABC MDR efflux pump could be considered a good target for the development of antibacterial drugs against this pathogen.
Keywords: Acanthamoeba polyphaga; Campylobacter jejuni; CmeB; antibiotic resistance; biofilm formation; host cell invasion; motility; multidrug efflux pumps; survival and multiplication.
Copyright © 2017 Vieira et al.
Figures


Similar articles
-
Emergence of a Potent Multidrug Efflux Pump Variant That Enhances Campylobacter Resistance to Multiple Antibiotics.mBio. 2016 Sep 20;7(5):e01543-16. doi: 10.1128/mBio.01543-16. mBio. 2016. PMID: 27651364 Free PMC article.
-
Mutation-based mechanism and evolution of the potent multidrug efflux pump RE-CmeABC in Campylobacter.Proc Natl Acad Sci U S A. 2024 Dec 17;121(51):e2415823121. doi: 10.1073/pnas.2415823121. Epub 2024 Nov 27. Proc Natl Acad Sci U S A. 2024. PMID: 39602248 Free PMC article.
-
Cryo-Electron Microscopy Structures of a Campylobacter Multidrug Efflux Pump Reveal a Novel Mechanism of Drug Recognition and Resistance.Microbiol Spectr. 2023 Aug 17;11(4):e0119723. doi: 10.1128/spectrum.01197-23. Epub 2023 Jun 8. Microbiol Spectr. 2023. PMID: 37289051 Free PMC article.
-
Campylobacter-Acanthamoeba interactions.Microbiology (Reading). 2015 May;161(Pt 5):933-947. doi: 10.1099/mic.0.000075. Epub 2015 Mar 10. Microbiology (Reading). 2015. PMID: 25757600 Review.
-
Resistance mechanisms in Campylobacter jejuni.Virulence. 2013 Apr 1;4(3):230-40. doi: 10.4161/viru.23753. Epub 2013 Feb 13. Virulence. 2013. PMID: 23406779 Free PMC article. Review.
Cited by
-
Waterborne Isolates of Campylobacter jejuni Are Able to Develop Aerotolerance, Survive Exposure to Low Temperature, and Interact With Acanthamoeba polyphaga.Front Microbiol. 2021 Oct 27;12:730858. doi: 10.3389/fmicb.2021.730858. eCollection 2021. Front Microbiol. 2021. PMID: 34777280 Free PMC article.
-
Adhesion of Campylobacter jejuni Is Increased in Association with Foodborne Bacteria.Microorganisms. 2020 Jan 31;8(2):201. doi: 10.3390/microorganisms8020201. Microorganisms. 2020. PMID: 32023990 Free PMC article.
-
Functional genomics study of Pseudomonas putida to determine traits associated with avoidance of a myxobacterial predator.Sci Rep. 2021 Aug 12;11(1):16445. doi: 10.1038/s41598-021-96046-8. Sci Rep. 2021. PMID: 34385565 Free PMC article.
-
Structure, Assembly, and Function of Tripartite Efflux and Type 1 Secretion Systems in Gram-Negative Bacteria.Chem Rev. 2021 May 12;121(9):5479-5596. doi: 10.1021/acs.chemrev.1c00055. Epub 2021 Apr 28. Chem Rev. 2021. PMID: 33909410 Free PMC article. Review.
-
The Impact of Protozoan Predation on the Pathogenicity of Vibrio cholerae.Front Microbiol. 2020 Jan 21;11:17. doi: 10.3389/fmicb.2020.00017. eCollection 2020. Front Microbiol. 2020. PMID: 32038597 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

