Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder
- PMID: 28916614
- PMCID: PMC5641681
- DOI: 10.15252/embj.201797568
Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder
Abstract
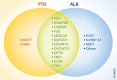
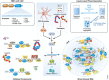
Frontotemporal dementia (FTD), the second most common form of dementia in people under 65 years of age, is characterized by progressive atrophy of the frontal and/or temporal lobes. FTD overlaps extensively with the motor neuron disease amyotrophic lateral sclerosis (ALS), especially at the genetic level. Both FTD and ALS can be caused by many mutations in the same set of genes; the most prevalent of these mutations is a GGGGCC repeat expansion in the first intron of C9ORF72 As shown by recent intensive studies, some key cellular pathways are dysregulated in the ALS-FTD spectrum disorder, including autophagy, nucleocytoplasmic transport, DNA damage repair, pre-mRNA splicing, stress granule dynamics, and others. These exciting advances reveal the complexity of the pathogenic mechanisms of FTD and ALS and suggest promising molecular targets for future therapeutic interventions in these devastating disorders.
Keywords: ALS; FTD; FUS; C9ORF72; TDP‐43.
© 2017 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet‐Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao FB (2013) Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC‐derived human neurons. Acta Neuropathol 126: 385–399 - PMC - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP‐43 is a component of ubiquitin‐positive tau‐negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

