Phosphoproteomics reveals that glycogen synthase kinase-3 phosphorylates multiple splicing factors and is associated with alternative splicing
- PMID: 28916722
- PMCID: PMC5672046
- DOI: 10.1074/jbc.M117.813527
Phosphoproteomics reveals that glycogen synthase kinase-3 phosphorylates multiple splicing factors and is associated with alternative splicing
Abstract
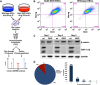
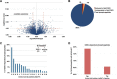
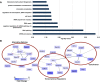
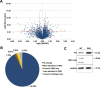
Glycogen synthase kinase-3 (GSK-3) is a constitutively active, ubiquitously expressed protein kinase that regulates multiple signaling pathways. In vitro kinase assays and genetic and pharmacological manipulations of GSK-3 have identified more than 100 putative GSK-3 substrates in diverse cell types. Many more have been predicted on the basis of a recurrent GSK-3 consensus motif ((pS/pT)XXX(S/T)), but this prediction has not been tested by analyzing the GSK-3 phosphoproteome. Using stable isotope labeling of amino acids in culture (SILAC) and MS techniques to analyze the repertoire of GSK-3-dependent phosphorylation in mouse embryonic stem cells (ESCs), we found that ∼2.4% of (pS/pT)XXX(S/T) sites are phosphorylated in a GSK-3-dependent manner. A comparison of WT and Gsk3a;Gsk3b knock-out (Gsk3 DKO) ESCs revealed prominent GSK-3-dependent phosphorylation of multiple splicing factors and regulators of RNA biosynthesis as well as proteins that regulate transcription, translation, and cell division. Gsk3 DKO reduced phosphorylation of the splicing factors RBM8A, SRSF9, and PSF as well as the nucleolar proteins NPM1 and PHF6, and recombinant GSK-3β phosphorylated these proteins in vitro RNA-Seq of WT and Gsk3 DKO ESCs identified ∼190 genes that are alternatively spliced in a GSK-3-dependent manner, supporting a broad role for GSK-3 in regulating alternative splicing. The MS data also identified posttranscriptional regulation of protein abundance by GSK-3, with ∼47 proteins (1.4%) whose levels increased and ∼78 (2.4%) whose levels decreased in the absence of GSK-3. This study provides the first unbiased analysis of the GSK-3 phosphoproteome and strong evidence that GSK-3 broadly regulates alternative splicing.
Keywords: SILAC; Wnt signaling; alternative splicing; embryonic stem cell; glycogen synthase kinase 3 (GSK-3); leukemia; lithium; phosphoproteomics; spliceosome.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Picton C., Woodgett J., Hemmings B., and Cohen P. (1982) Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle: phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 150, 191–196 - PubMed
-
- Hoeflich K. P., Luo J., Rubie E. A., Tsao M. S., Jin O., and Woodgett J. R. (2000) Requirement for glycogen synthase kinase-3β in cell survival and NF- κB activation. Nature 406, 86–90 - PubMed
-
- Liu K. J., Arron J. R., Stankunas K., Crabtree G. R., and Longaker M. T. (2007) Chemical rescue of cleft palate and midline defects in conditional GSK-3β mice. Nature 446, 79–82 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

