The catalytic subunit of DNA polymerase δ inhibits γTuRC activity and regulates Golgi-derived microtubules
- PMID: 28916777
- PMCID: PMC5601897
- DOI: 10.1038/s41467-017-00694-2
The catalytic subunit of DNA polymerase δ inhibits γTuRC activity and regulates Golgi-derived microtubules
Abstract
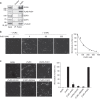
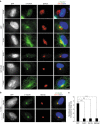
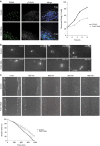
γ-Tubulin ring complexes (γTuRCs) initiate microtubule growth and mediate microtubule attachment at microtubule-organizing centers, such as centrosomes and the Golgi complex. However, the mechanisms that control γTuRC-mediated microtubule nucleation have remained mostly unknown. Here, we show that the DNA polymerase δ catalytic subunit (PolD1) binds directly to γTuRCs and potently inhibits γTuRC-mediated microtubule nucleation. Whereas PolD1 depletion through RNA interference does not influence centrosome-based microtubule growth, the depletion augments microtubule nucleation at the Golgi complex. Conversely, PolD1 overexpression inhibits Golgi-based microtubule nucleation. Golgi-derived microtubules are required for the assembly and maintenance of the proper Golgi structure, and we found that alteration of PolD1 levels affects Golgi structural organization. Moreover, suppression of PolD1 expression impairs Golgi reassembly after nocodazole-induced disassembly and causes defects in Golgi reorientation and directional cell migration. Collectively, these results reveal a mechanism that controls noncentrosomal γTuRC activity and regulates the organization of Golgi-derived microtubules.Microtubule organization requires γ-tubulin ring complexes (γTuRCs), but the mechanisms that control γTuRC-mediated microtubule nucleation are unclear. Here the authors show that the DNA polymerase δ catalytic subunit controls noncentrosomal γTuRC activity and regulates the organization of Golgi-derived microtubules.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Detection and Analysis of Microtubule Nucleator γ-Tubulin Ring Complex.Methods Mol Biol. 2023;2557:543-558. doi: 10.1007/978-1-0716-2639-9_32. Methods Mol Biol. 2023. PMID: 36512236
-
Autoinhibitory mechanism controls binding of centrosomin motif 1 to γ-tubulin ring complex.J Cell Biol. 2023 Jul 3;222(7):e202007101. doi: 10.1083/jcb.202007101. Epub 2023 May 22. J Cell Biol. 2023. PMID: 37213089 Free PMC article.
-
The conserved centrosomin motif, γTuNA, forms a dimer that directly activates microtubule nucleation by the γ-tubulin ring complex (γTuRC).Elife. 2022 Dec 14;11:e80053. doi: 10.7554/eLife.80053. Elife. 2022. PMID: 36515268 Free PMC article.
-
Regulation of microtubule nucleation mediated by γ-tubulin complexes.Protoplasma. 2017 May;254(3):1187-1199. doi: 10.1007/s00709-016-1070-z. Epub 2017 Jan 10. Protoplasma. 2017. PMID: 28074286 Review.
-
The augmin connection in the geometry of microtubule networks.Curr Biol. 2015 Mar 30;25(7):R294-9. doi: 10.1016/j.cub.2015.02.006. Curr Biol. 2015. PMID: 25829017 Review.
Cited by
-
Microtubular and Nuclear Functions of γ-Tubulin: Are They LINCed?Cells. 2019 Mar 19;8(3):259. doi: 10.3390/cells8030259. Cells. 2019. PMID: 30893853 Free PMC article. Review.
-
The dual role of the centrosome in organizing the microtubule network in interphase.EMBO Rep. 2018 Nov;19(11):e45942. doi: 10.15252/embr.201845942. Epub 2018 Sep 17. EMBO Rep. 2018. PMID: 30224411 Free PMC article.
-
Dissecting the novel partners of nuclear c-Raf and its role in all-trans retinoic acid (ATRA)-induced myeloblastic leukemia cells differentiation.Exp Cell Res. 2020 Sep 1;394(1):111989. doi: 10.1016/j.yexcr.2020.111989. Epub 2020 Apr 10. Exp Cell Res. 2020. PMID: 32283065 Free PMC article.
-
Nek2-mediated GAS2L1 phosphorylation and centrosome-linker disassembly induce centrosome disjunction.J Cell Biol. 2020 May 4;219(5):e201909094. doi: 10.1083/jcb.201909094. J Cell Biol. 2020. PMID: 32289147 Free PMC article.
-
The prognostic and neuroendocrine implications of SLC25A29-mediated biomass signature in prostate cancer.Geroscience. 2025 Jun;47(3):4711-4732. doi: 10.1007/s11357-025-01538-4. Epub 2025 Jan 31. Geroscience. 2025. PMID: 39890746 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

