Preserved immune functionality and high CMV-specific T-cell responses in HIV-infected individuals with poor CD4+ T-cell immune recovery
- PMID: 28916780
- PMCID: PMC5601464
- DOI: 10.1038/s41598-017-12013-2
Preserved immune functionality and high CMV-specific T-cell responses in HIV-infected individuals with poor CD4+ T-cell immune recovery
Abstract
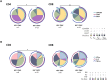
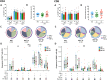
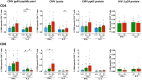
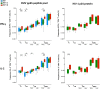
Poor CD4+ T-cell recovery after cART has been associated with skewed T-cell maturation, inflammation and immunosenescence; however, T-cell functionality in those individuals has not been fully characterized. In the present study, we assessed T-cell function by assessing cytokine production after polyclonal, CMV and HIV stimulations of T-cells from ART-suppressed HIV-infected individuals with CD4+ T-cell counts >350 cells/μL (immunoconcordants) or <350 cells/μL (immunodiscordants). A group of HIV-uninfected individuals were also included as controls. Since CMV co-infection significantly affected T-cell maturation and polyfunctionality, only CMV+ individuals were analyzed. Despite their reduced and skewed CD4+ T-cell compartment, immunodiscordant individuals showed preserved polyclonal and HIV-specific responses. However, CMV response in immunodiscordant participants was significantly different from immunoconcordant or HIV-seronegative individuals. In immunodiscordant subjects, the magnitude of IFN-γ+ CD8+ and IL-2+ CD4+ T-cells in response to CMV was higher and differently associated with the CD4+ T-cell maturation profile., showing an increased frequency of naïve, central memory and EMRA CMV-specific CD4+ T-cells. In conclusion, CD4+ and CD8+ T-cell polyfunctionality was not reduced in immunodiscordant individuals, although heightened CMV-specific immune responses, likely related to subclinical CMV reactivations, may be contributing to the skewed T-cell maturation and the higher risk of clinical progression observed in those individuals.
Conflict of interest statement
E.G.M., M.M., E.G., V.U., J.P., J.B., E.N. and C.C. report no disclosures. B.C. have served as a consultant to and/or have received research grant support from Gilead, Janssen, MSD, ViiV, BMS.
Figures
Similar articles
-
Cytomegalovirus-specific T-cells are associated with immune senescence, but not with systemic inflammation, in people living with HIV.Sci Rep. 2018 Feb 28;8(1):3778. doi: 10.1038/s41598-018-21347-4. Sci Rep. 2018. PMID: 29491459 Free PMC article.
-
Elevated humoral response to cytomegalovirus in HIV-infected individuals with poor CD4+ T-cell immune recovery.PLoS One. 2017 Sep 21;12(9):e0184433. doi: 10.1371/journal.pone.0184433. eCollection 2017. PLoS One. 2017. PMID: 28934217 Free PMC article.
-
CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection.Clin Infect Dis. 2016 Feb 1;62(3):392-6. doi: 10.1093/cid/civ840. Epub 2015 Sep 23. Clin Infect Dis. 2016. PMID: 26400999 Free PMC article.
-
Cytomegalovirus and HIV Persistence: Pouring Gas on the Fire.AIDS Res Hum Retroviruses. 2017 Nov;33(S1):S23-S30. doi: 10.1089/aid.2017.0145. AIDS Res Hum Retroviruses. 2017. PMID: 29140108 Free PMC article. Review.
-
Cytomegalovirus as an Uninvited Guest in the Response to Vaccines in People Living with HIV.Viruses. 2021 Jun 29;13(7):1266. doi: 10.3390/v13071266. Viruses. 2021. PMID: 34209711 Free PMC article. Review.
Cited by
-
PSGL-1, a Strategic Biomarker for Pathological Conditions in HIV Infection: A Hypothesis Review.Viruses. 2023 Oct 31;15(11):2197. doi: 10.3390/v15112197. Viruses. 2023. PMID: 38005875 Free PMC article. Review.
-
The forces driving clonal expansion of the HIV-1 latent reservoir.Virol J. 2020 Jan 7;17(1):4. doi: 10.1186/s12985-019-1276-8. Virol J. 2020. PMID: 31910871 Free PMC article. Review.
-
Cytomegalovirus-specific T-cells are associated with immune senescence, but not with systemic inflammation, in people living with HIV.Sci Rep. 2018 Feb 28;8(1):3778. doi: 10.1038/s41598-018-21347-4. Sci Rep. 2018. PMID: 29491459 Free PMC article.
-
NK Count and Natural Cytotoxicity in Immune Nonresponders Versus Responders Living With HIV.J Med Virol. 2025 Feb;97(2):e70170. doi: 10.1002/jmv.70170. J Med Virol. 2025. PMID: 39868926 Free PMC article.
-
HIV Expression in Infected T Cell Clones.Viruses. 2024 Jan 11;16(1):108. doi: 10.3390/v16010108. Viruses. 2024. PMID: 38257808 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

