In Vitro and In Vivo Biocompatibility Evaluation of Polyallylamine and Macromolecular Heparin Conjugates Modified Alginate Microbeads
- PMID: 28916826
- PMCID: PMC5600981
- DOI: 10.1038/s41598-017-11989-1
In Vitro and In Vivo Biocompatibility Evaluation of Polyallylamine and Macromolecular Heparin Conjugates Modified Alginate Microbeads
Abstract
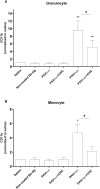
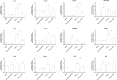
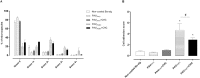
Host reactivity to biocompatible immunoisolation devices is a major challenge for cellular therapies, and a human screening model would be of great value. We designed new types of surface modified barium alginate microspheres, and evaluated their inflammatory properties using human whole blood, and the intraperitoneal response after three weeks in Wistar rats. Microspheres were modified using proprietary polyallylamine (PAV) and coupled with macromolecular heparin conjugates (Corline Heparin Conjugate, CHC). The PAV-CHC strategy resulted in uniform and stable coatings with increased anti-clot activity and low cytotoxicity. In human whole blood, PAV coating at high dose (100 µg/ml) induced elevated complement, leukocyte CD11b and inflammatory mediators, and in Wistar rats increased fibrotic overgrowth. Coating of high dose PAV with CHC significantly reduced these responses. Low dose PAV (10 µg/ml) ± CHC and unmodified alginate microbeads showed low responses. That the human whole blood inflammatory reactions paralleled the host response shows a link between inflammatory potential and initial fibrotic response. CHC possessed anti-inflammatory activity, but failed to improve overall biocompatibility. We conclude that the human whole blood assay is an efficient first-phase screening model for inflammation, and a guiding tool in development of new generation microspheres for cell encapsulation therapy.
Conflict of interest statement
One of the authors, RL, carries out contract work for Corline System AB, which supplied us with a number of the reagents used, for example, heparin conjugate.
Figures




Similar articles
-
Beneficial effects of coating alginate microcapsules with macromolecular heparin conjugates-in vitro and in vivo study.Tissue Eng Part A. 2014 Jan;20(1-2):324-34. doi: 10.1089/ten.TEA.2013.0254. Epub 2013 Oct 2. Tissue Eng Part A. 2014. PMID: 23971677
-
Sulfated alginate microspheres associate with factor H and dampen the inflammatory cytokine response.Acta Biomater. 2016 Sep 15;42:180-188. doi: 10.1016/j.actbio.2016.06.015. Epub 2016 Jun 11. Acta Biomater. 2016. PMID: 27296843
-
Pericapsular fibrotic overgrowth mitigated in immunocompetent mice through microbead formulations based on sulfated or intermediate G alginates.Acta Biomater. 2022 Jan 1;137:172-185. doi: 10.1016/j.actbio.2021.10.004. Epub 2021 Oct 8. Acta Biomater. 2022. PMID: 34634509
-
Alginate microbeads are complement compatible, in contrast to polycation containing microcapsules, as revealed in a human whole blood model.Acta Biomater. 2011 Jun;7(6):2566-78. doi: 10.1016/j.actbio.2011.03.011. Epub 2011 Mar 12. Acta Biomater. 2011. PMID: 21402181
-
Advances in biocompatibility and physico-chemical characterization of microspheres for cell encapsulation.Adv Drug Deliv Rev. 2014 Apr;67-68:111-30. doi: 10.1016/j.addr.2013.07.010. Epub 2013 Jul 20. Adv Drug Deliv Rev. 2014. PMID: 23876549 Review.
Cited by
-
Gallic Acid Loaded Alginate-Gelatin Beads for Potential Bone Tissue Engineering Applications.Biopolymers. 2025 Jul;116(4):e70033. doi: 10.1002/bip.70033. Biopolymers. 2025. PMID: 40467047 Free PMC article.
-
MS-proteomics provides insight into the host responses towards alginate microspheres.Mater Today Bio. 2022 Nov 11;17:100490. doi: 10.1016/j.mtbio.2022.100490. eCollection 2022 Dec 15. Mater Today Bio. 2022. PMID: 36420052 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

