Early assessment of response to induction therapy in acute myeloid leukemia using 18F-FLT PET/CT
- PMID: 28916904
- PMCID: PMC5602811
- DOI: 10.1186/s13550-017-0326-8
Early assessment of response to induction therapy in acute myeloid leukemia using 18F-FLT PET/CT
Abstract
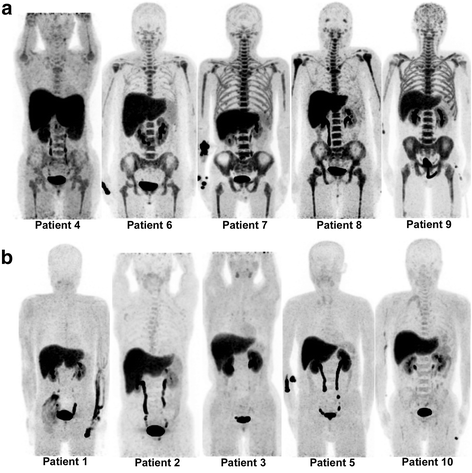
Background: We evaluated the suitability of 18F-fluorodeoxythymidine (18F-FLT) positron emission tomography (PET)/computed tomography (CT) for assessment of the early response to induction therapy and its value for predicting clinical outcome in patients with acute myeloid leukemia (AML). Adult patients who had histologically confirmed AML and received induction therapy were enrolled. All patients underwent 18F-FLT PET/CT after completion of induction. PET/CT images were visually and quantitatively assessed. Cases with intensely increased bone marrow uptake in more than one third of the long bones and throughout the central skeleton were interpreted as PET-positive for resistant disease (RD). PET results were compared to the clinical response and outcome.
Results: In visual PET analysis of 10 eligible patients (7 male, 3 female; median age 58 years), 5 patients were interpreted as being PET-positive and 5 as PET-negative. Standardized uptake values were significantly different between PET-positive and PET-negative groups. Eight of 10 patients achieved clinical complete remission (CR)/CR with incomplete blood count recovery (CRi). Five CR/CRi patients had PET-negative findings, but 3 CR patients had PET-positive findings. Both of the RD patients had PET-positive findings. During follow-up, 2 CR patients with PET-positive findings relapsed, or were strongly suspected of relapse, 4 months after consolidation.
Conclusion: 18F-FLT PET/CT after induction therapy showed good sensitivity and negative-predictive value for evaluating RD in patients with AML. This preliminary study suggests that 18F-FLT PET/CT may be valuable as a noninvasive tool for early assessment of the response to treatment and may provide prognostic value for survival in patients with AML.
Keywords: 18F-FLT; Acute myeloid leukemia; Bone marrow; Induction therapy; PET; Response assessment.
Conflict of interest statement
Ethics approval and consent to participate
This study was performed in accordance with the approved guidelines of St. Vincent’s Hospital’s institutional review board. Written consent was obtained from all participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 trial. Blood. 2003;101:64–70. doi: 10.1182/blood-2002-02-0532. - DOI - PubMed
-
- Rowe JM, Kim HT, Cassileth PA, Lazarus HM, Litzow MR, Wiernik PH, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116:5012–5021. doi: 10.1002/cncr.25263. - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): acute myeloid leukemia (Version 2.2017). http://www.nccn.org/professionals/physiciangls/pdf/aml.pdf. Accessed 9 April 2017.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

