Coupled reactions by coupled enzymes: alcohol to lactone cascade with alcohol dehydrogenase-cyclohexanone monooxygenase fusions
- PMID: 28916997
- PMCID: PMC5624969
- DOI: 10.1007/s00253-017-8501-4
Coupled reactions by coupled enzymes: alcohol to lactone cascade with alcohol dehydrogenase-cyclohexanone monooxygenase fusions
Abstract
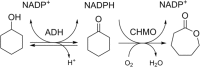
The combination of redox enzymes for redox-neutral cascade reactions has received increasing appreciation. An example is the combination of an alcohol dehydrogenase (ADH) with a cyclohexanone monooxygenase (CHMO). The ADH can use NADP+ to oxidize cyclohexanol to form cyclohexanone and NADPH. Both products are then used by CHMO to produce ε-caprolactone. In this study, these two redox-complementary enzymes were fused, to create a self-sufficient bifunctional enzyme that can convert alcohols to esters or lactones. Three different ADH genes were fused to a gene coding for a thermostable CHMO, in both orientations (ADH-CHMO and CHMO-ADH). All six fusion enzymes could be produced and purified. For two of the three ADHs, we found a clear difference between the two orientations: one that showed the expected ADH activity, and one that showed low to no activity. The ADH activity of each fusion enzyme correlated with its oligomerization state. All fusions retained CHMO activity, and stability was hardly affected. The TbADH-TmCHMO fusion was selected to perform a cascade reaction, producing ε-caprolactone from cyclohexanol. By circumventing substrate and product inhibition, a > 99% conversion of 200 mM cyclohexanol could be achieved in 24 h, with > 13,000 turnovers per fusion enzyme molecule.
Keywords: Alcohol dehydrogenase; Cascade; Cyclohexanone monooxygenase; Enzyme fusion.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals by any of the authors.
Figures

Similar articles
-
Enzyme cascade converting cyclohexanol into ε-caprolactone coupled with NADPH recycling using surface displayed alcohol dehydrogenase and cyclohexanone monooxygenase on E. coli.Microb Biotechnol. 2022 Aug;15(8):2235-2249. doi: 10.1111/1751-7915.14062. Epub 2022 Apr 27. Microb Biotechnol. 2022. PMID: 35478318 Free PMC article.
-
Co-expression of an alcohol dehydrogenase and a cyclohexanone monooxygenase for cascade reactions facilitates the regeneration of the NADPH cofactor.Enzyme Microb Technol. 2018 Jan;108:53-58. doi: 10.1016/j.enzmictec.2017.09.003. Epub 2017 Sep 11. Enzyme Microb Technol. 2018. PMID: 29108627
-
A self-sufficient Baeyer-Villiger biocatalysis system for the synthesis of ɛ-caprolactone from cyclohexanol.Enzyme Microb Technol. 2013 Sep 10;53(4):283-7. doi: 10.1016/j.enzmictec.2013.01.007. Epub 2013 Jan 25. Enzyme Microb Technol. 2013. PMID: 23931695
-
Use of the anti-Prelog stereospecific alcohol dehydrogenase from Leifsonia and Pseudomonas for producing chiral alcohols.Appl Microbiol Biotechnol. 2014 May;98(9):3889-904. doi: 10.1007/s00253-014-5619-5. Epub 2014 Mar 11. Appl Microbiol Biotechnol. 2014. PMID: 24615386 Review.
-
Impact and relevance of alcohol dehydrogenase enantioselectivities on biotechnological applications.Appl Microbiol Biotechnol. 2020 Apr;104(7):2897-2909. doi: 10.1007/s00253-020-10440-2. Epub 2020 Feb 15. Appl Microbiol Biotechnol. 2020. PMID: 32060695 Review.
Cited by
-
Toward Upscaled Biocatalytic Preparation of Lactone Building Blocks for Polymer Applications.Org Process Res Dev. 2018 Jul 20;22(7):803-812. doi: 10.1021/acs.oprd.8b00079. Epub 2018 Jun 12. Org Process Res Dev. 2018. PMID: 30271110 Free PMC article. Review.
-
Enzyme cascade converting cyclohexanol into ε-caprolactone coupled with NADPH recycling using surface displayed alcohol dehydrogenase and cyclohexanone monooxygenase on E. coli.Microb Biotechnol. 2022 Aug;15(8):2235-2249. doi: 10.1111/1751-7915.14062. Epub 2022 Apr 27. Microb Biotechnol. 2022. PMID: 35478318 Free PMC article.
-
Stabilization of cyclohexanone monooxygenase by computational and experimental library design.Biotechnol Bioeng. 2019 Sep;116(9):2167-2177. doi: 10.1002/bit.27022. Epub 2019 Jun 24. Biotechnol Bioeng. 2019. PMID: 31124128 Free PMC article.
-
Design of Artificial Alcohol Oxidases: Alcohol Dehydrogenase-NADPH Oxidase Fusions for Continuous Oxidations.Chembiochem. 2019 Jan 2;20(1):51-56. doi: 10.1002/cbic.201800421. Epub 2018 Oct 4. Chembiochem. 2019. PMID: 30184296 Free PMC article.
-
Multidimensional Engineering of Escherichia coli for Efficient Adipic Acid Synthesis From Cyclohexane.Adv Sci (Weinh). 2025 Apr;12(14):e2411938. doi: 10.1002/advs.202411938. Epub 2025 Feb 17. Adv Sci (Weinh). 2025. PMID: 39960345 Free PMC article.
References
-
- Bornadel A, Hatti-Kaul R, Hollmann F, Kara S. Enhancing the productivity of the bi-enzymatic convergent cascade for ɛ-caprolactone synthesis through design of experiments and a biphasic system. Tetrahedron. 2016;72(46):7222–7228. doi: 10.1016/j.tet.2015.11.054. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

