Ultrasound-assisted liposuction provides a source for functional adipose-derived stromal cells
- PMID: 28917626
- PMCID: PMC5723208
- DOI: 10.1016/j.jcyt.2017.07.013
Ultrasound-assisted liposuction provides a source for functional adipose-derived stromal cells
Abstract
Background aims: Regenerative medicine employs human mesenchymal stromal cells (MSCs) for their multi-lineage plasticity and their pro-regenerative cytokine secretome. Adipose-derived mesenchymal stromal cells (ASCs) are concentrated in fat tissue, and the ease of harvest via liposuction makes them a particularly interesting cell source. However, there are various liposuction methods, and few have been assessed regarding their impact on ASC functionality. Here we study the impact of the two most popular ultrasound-assisted liposuction (UAL) devices currently in clinical use, VASER (Solta Medical) and Lysonix 3000 (Mentor) on ASCs.
Methods: After lipoaspirate harvest and processing, we sorted for ASCs using fluorescent-assisted cell sorting based on an established surface marker profile (CD34+CD31-CD45-). ASC yield, viability, osteogenic and adipogenic differentiation capacity and in vivo regenerative performance were assessed.
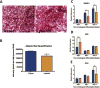
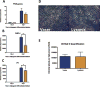
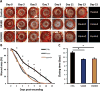
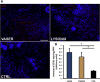
Results: Both UAL samples demonstrated equivalent ASC yield and viability. VASER UAL ASCs showed higher osteogenic and adipogenic marker expression, but a comparable differentiation capacity was observed. Soft tissue healing and neovascularization were significantly enhanced via both UAL-derived ASCs in vivo, and there was no significant difference between the cell therapy groups.
Conclusions: Taken together, our data suggest that UAL allows safe and efficient harvesting of the mesenchymal stromal cellular fraction of adipose tissue and that cells harvested via this approach are suitable for cell therapy and tissue engineering applications.
Keywords: ASCs; Lysonix; VASER; adipose-derived stromal cells; adult mesenchymal stromal cells; cell therapy; regenerative medicine; ultrasound-assisted liposuction.
Copyright © 2017 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DD and GCG are listed on the patent “Efficient Stem Cell Delivery Into Biomaterials Using a Novel Capillary Driven Encapsulation Technique” and GCG is listed on the patent “Intelligent Biodegradable Pullulan Regenerative Matrix for Tissue Engineering” assigned to Stanford University. ZNM, AL, MMA, EAB, DA, AJW, MSH, GGW, KSH, AFS, HGM, MTL and DCW have no potential conflicts of interest, affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed herein.
Figures

Similar articles
-
Ultrasound-Assisted Liposuction Does Not Compromise the Regenerative Potential of Adipose-Derived Stem Cells.Stem Cells Transl Med. 2016 Feb;5(2):248-57. doi: 10.5966/sctm.2015-0064. Epub 2015 Dec 23. Stem Cells Transl Med. 2016. PMID: 26702129 Free PMC article.
-
Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine.Stem Cells Transl Med. 2013 Oct;2(10):808-17. doi: 10.5966/sctm.2012-0183. Epub 2013 Sep 9. Stem Cells Transl Med. 2013. PMID: 24018794 Free PMC article.
-
Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells.J Transl Med. 2016 May 6;14(1):126. doi: 10.1186/s12967-016-0881-1. J Transl Med. 2016. PMID: 27153799 Free PMC article.
-
Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells.Int J Mol Sci. 2018 Jul 27;19(8):2200. doi: 10.3390/ijms19082200. Int J Mol Sci. 2018. PMID: 30060511 Free PMC article. Review.
-
Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review.Curr Stem Cell Res Ther. 2017;12(6):484-492. doi: 10.2174/1574888X11666160429122133. Curr Stem Cell Res Ther. 2017. PMID: 27133085
Cited by
-
Extracellular Vesicles of Mesenchymal Stromal Cells Can be Taken Up by Microglial Cells and Partially Prevent the Stimulation Induced by β-amyloid.Stem Cell Rev Rep. 2022 Mar;18(3):1113-1126. doi: 10.1007/s12015-021-10261-4. Epub 2022 Jan 26. Stem Cell Rev Rep. 2022. PMID: 35080744 Free PMC article.
-
Comparison between Intra-Articular Injection of Infrapatellar Fat Pad (IPFP) Cell Concentrates and IPFP-Mesenchymal Stem Cells (MSCs) for Cartilage Defect Repair of the Knee Joint in Rabbits.Stem Cells Int. 2021 Jul 27;2021:9966966. doi: 10.1155/2021/9966966. eCollection 2021. Stem Cells Int. 2021. PMID: 34367294 Free PMC article.
-
The Short-Term Effect of VASER Assisted Liposuction on Lipid Profile.Aesthetic Plast Surg. 2023 Apr;47(2):685-689. doi: 10.1007/s00266-022-03098-w. Epub 2022 Dec 6. Aesthetic Plast Surg. 2023. PMID: 36474015
-
Fibrin Glue Enhances Adipose-Derived Stromal Cell Cytokine Secretion and Survival Conferring Accelerated Diabetic Wound Healing.Stem Cells Int. 2018 Dec 19;2018:1353085. doi: 10.1155/2018/1353085. eCollection 2018. Stem Cells Int. 2018. PMID: 30662467 Free PMC article.
-
Musculoskeletal tissue engineering: Adipose derived stromal cell implementation for the treatment of osteoarthritis.Biomaterials. 2022 Jul;286:121544. doi: 10.1016/j.biomaterials.2022.121544. Epub 2022 May 6. Biomaterials. 2022. PMID: 35633592 Free PMC article. Review.
References
-
- Sterodimas A, et al. Thirtyfour years of liposuction: past, present and future. Eur Rev Med Pharmacol Sci. 2012;16(3):393–406. - PubMed
-
- Fischer G. Liposculpture: the “correct” history of liposuction. Part I. Dermatologic Surgery. 1990;16(12):1087–1089. - PubMed
-
- Berry MG, Davies D. Liposuction: a review of principles and techniques. J Plast Reconstr Aesthet Surg. 2011;64(8):985–92. - PubMed
-
- Zocchi M. Ultrasonic liposculpturing. Aesthetic plastic surgery. 1992;16(4):287–298. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

